All Photos(1)
About This Item
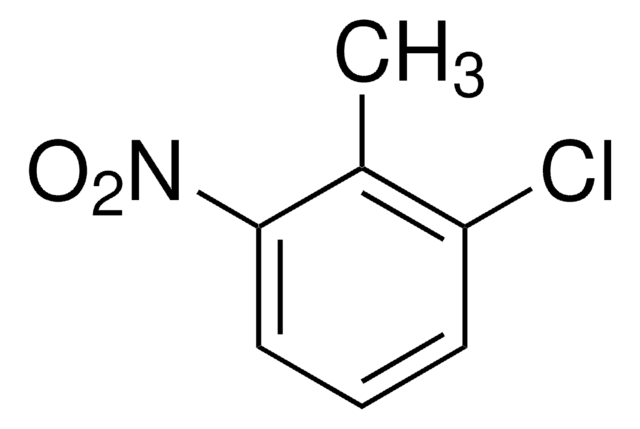
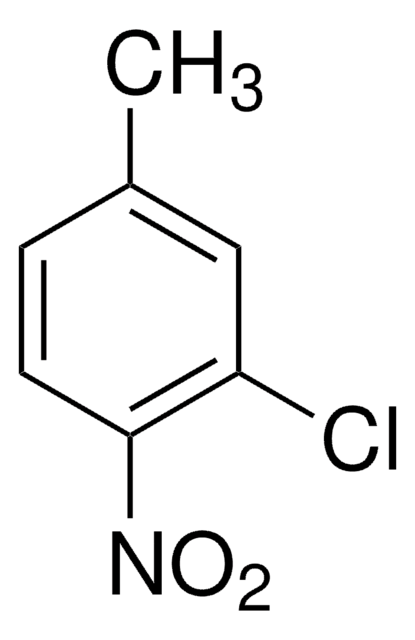
Linear Formula:
CH3C6H3(NO2)Cl
CAS Number:
Molecular Weight:
171.58
Beilstein:
2046651
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
239-240 °C/718 mmHg (lit.)
mp
34-38 °C (lit.)
SMILES string
Cc1ccc(Cl)cc1[N+]([O-])=O
InChI
1S/C7H6ClNO2/c1-5-2-3-6(8)4-7(5)9(10)11/h2-4H,1H3
InChI key
SQFLFRQWPBEDHM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Chloro-2-nitrotoluene can be used in the synthesis of indigo dye.
Biochem/physiol Actions
4-Chloro-2-nitrotoluene is the starting reagent for synthesis of tricyclic indole-2-carboxylic acids, a potential NMDA-glycine antagonists.
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
248.0 °F - closed cup
Flash Point(C)
120 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Thanasis Karapanayiotis et al.
The Analyst, 129(7), 613-618 (2004-06-24)
The Raman and electron impact mass spectra of synthetic indigo and its four 6,6'-dihalogeno analogues are reported and discussed. The influence of varying the halogen on these Raman spectra is considered. Particular emphasis is laid on distinguishing indigo from 6,6'-dibromoindigo
S Katayama et al.
The Journal of organic chemistry, 66(10), 3474-3483 (2001-05-12)
The practical synthesis of a series of tricyclic indole-2-carboxylic acids, 7-chloro-3-arylaminocarbonylmethyl-1,3,4,5-tetrahydrobenz[cd]indole-2-carboxylic acids, as a new class of potent NMDA-glycine antagonists is described. The synthetic route to the key intermediate 12a comprises a regioselective iodination of 4-chloro-2-nitrotoluene, modified Reissert indole synthesis
D de Almeida Azevedo et al.
Journal of chromatography. A, 879(1), 13-26 (2000-06-28)
Gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-atmospheric pressure chemical ionization mass spectrometry (LC-APCI-MS) were optimized and applied for the trace-level determination of 42 priority pesticides and 33 priority organic pollutants from European Union Directive EC 76/464. First, off-line solid-phase extraction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service