About This Item
Recommended Products
grade
analytical standard
Quality Level
CofA
current certificate can be downloaded
packaging
ampule of 5000 mg
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
bp
298 °C (lit.)
mp
111-114 °C (lit.)
application(s)
environmental
format
neat
storage temp.
2-30°C
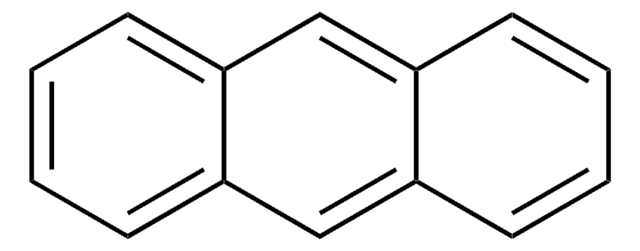
SMILES string
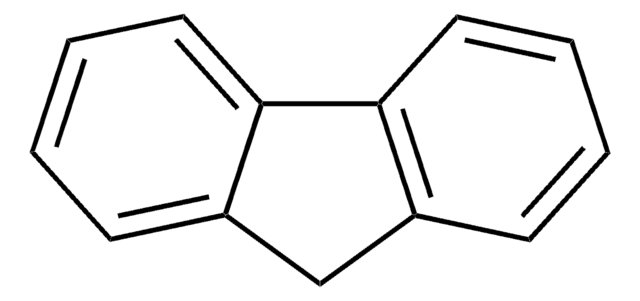
C1c2ccccc2-c3ccccc13
InChI
1S/C13H10/c1-3-7-12-10(5-1)9-11-6-2-4-8-13(11)12/h1-8H,9H2
InChI key
NIHNNTQXNPWCJQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
303.8 °F - closed cup
Flash Point(C)
151.0 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
GC Analysis of PAHs on SLB®-5ms
US EPA Method 610 describes the analysis of polynuclear aromatic hydrocarbons (commonly referred to as PAHs or PNAs) by both HPLC and GC.
HPLC Analysis of PAHs on SUPELCOSIL™ LC-PAH
GC Analysis of Polynuclear Aromatic Hydrocarbons (PAHs) in Salmon on SPB®-608 (20 m x 0.18 mm I.D., 0.18 µm) after QuEChERS Cleanup using Supel™ QuE Z-Sep, Fast GC Analysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service