S6322
Sigma Adjuvant System®
oil
Synonym(s):
Adjuvant System, Sigma Adjuvant
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
12352203
NACRES:
NA.46
Recommended Products
Application
Adjuvanticity Assay: EIA titer for test mice is at least 2X greater that that of control mice.
Biochem/physiol Actions
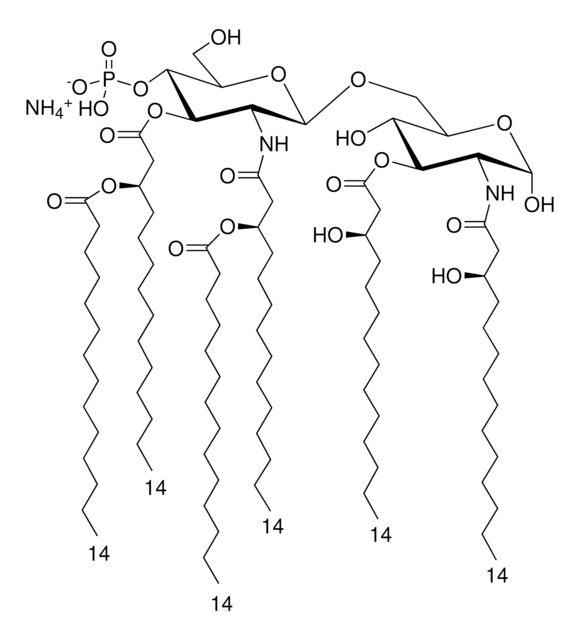
Sigma Adjuvant System is a stable oil-in-water emulsion that may be used as an alternative to the classical Freund′s water-in-oil emulsions. It is derived from bacterial and mycobacterial cell wall components that provide potent stimulus to the immune system.
Physical form
Each vial contains 0.5 mg Monophosphoryl Lipid A (detoxified endotoxin) from Salmonella minnesota and 0.5 mg synthetic Trehalose Dicorynomycolate in 2% oil (squalene)-Tween® 80-water.
Legal Information
Sigma Adjuvant System is a registered trademark of Merck KGaA, Darmstadt, Germany
TWEEN is a registered trademark of Croda International PLC
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nadja Thönes et al.
Journal of virology, 82(11), 5472-5485 (2008-04-04)
Capsomeres are considered to be an alternative to viruslike particle (VLP)-based vaccines as they can be produced in prokaryotic expression systems. So far, no detailed side-by-side comparison of VLPs and capsomeres has been performed. In the present study, we immunized
Active immunization of hamsters against Clostridium difficile infection using surface-layer protein.
Déirdre B Ní Eidhin et al.
FEMS immunology and medical microbiology, 52(2), 207-218 (2007-12-21)
Clostridium difficile is the leading cause of infectious antibiotic-associated diarrhoea, particularly among the elderly. Its surface-layer protein (SLP) was tested as a vaccine component in a series of immunization and challenge experiments with Golden Syrian hamsters, combined with different systemic
Avi-Hai Hovav et al.
Infection and immunity, 73(1), 250-257 (2004-12-25)
In this study, we examined the immunogenicity and protective efficacy of six immunodominant Mycobacterium tuberculosis recombinant antigens (85B, 38kDa, ESAT-6, CFP21, Mtb8.4, and 16kDa) in a multivalent vaccine preparation (6Ag). Gamma interferon (IFN-gamma) and monophosphoryl lipid A-trehalose dicorynomycolate (Ribi) adjuvant
Gabriel Ozorowski et al.
Journal of virology, 92(13) (2018-04-20)
Native-like, soluble, recombinant SOSIP trimers of various designs and based on several env genes of human immunodeficiency virus type 1 (HIV-1) are being tested as immunogens in different animal models. These experiments almost always involve coformulating the trimers with an
Development of monoclonal antibodies specific for Ricinus agglutinins
Brandon DL and Hernlem BJ
Food and agricultural immunology, 20(1), 11-22 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service