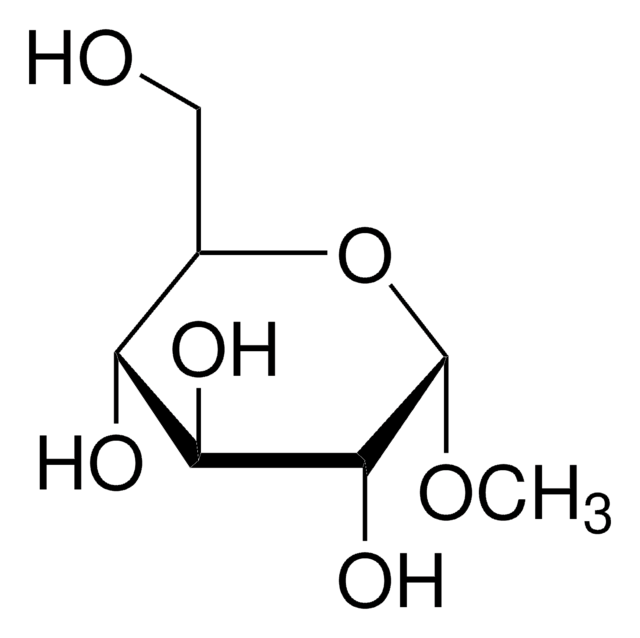
O0630
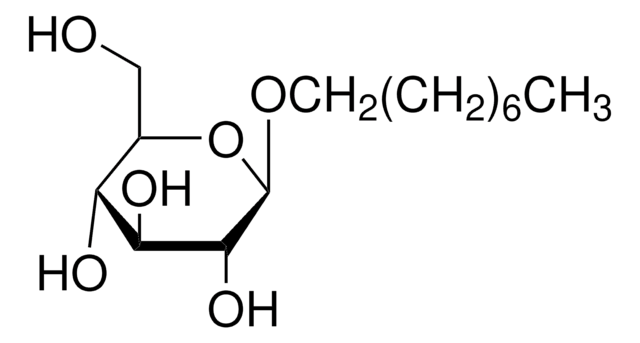
Octyl α-D-glucopyranoside
≥98% (GC)
Synonym(s):
n-Octyl α-glucoside
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C14H28O6
CAS Number:
Molecular Weight:
292.37
Beilstein:
4232620
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
description
non-ionic
Assay
≥98% (GC)
mol wt
292.37 g/mol
storage temp.
−20°C
SMILES string
CCCCCCCCO[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O
InChI
1S/C14H28O6/c1-2-3-4-5-6-7-8-19-14-13(18)12(17)11(16)10(9-15)20-14/h10-18H,2-9H2,1H3/t10-,11-,12+,13-,14+/m1/s1
InChI key
HEGSGKPQLMEBJL-RGDJUOJXSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Octyl α-D-glucopyranoside has been used in a study to assess mesogenic structures with interdigitizing alkyl chains. It has also been used in a study to investigate a recirculation procedure involving water removal by product crystallization.
Biochem/physiol Actions
Dialyzable nonionic detergent, suitable for the solubilization and purification of membrane proteins. Can be used for the crystallization of membrane proteins.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Efficient Preparation of Octyl α-D-Glucopyranoside Monohydrate: A Recirculation Procedure Involving Water Removal by Product Crystallisation
Straathof, A., et al.
Starch/Staerke, 40, 229-234 (1988)
Jung-Deog Lee et al.
Organic letters, 7(6), 963-966 (2005-03-12)
[structure: see text] Porphyrin-based molecularly imprinted polymers (MIPs) were prepared for carbohydrate recognition. A urea-appended porphyrin functional monomer was utilized to provide complementary functionality and quality binding sites throughout the polymer. Each porphyrin-based polymer demonstrates high affinity and differential selectivity
G A Jeffrey et al.
Carbohydrate research, 169, 1-11 (1987-11-15)
The crystal structure of octyl alpha-D-glucopyranoside monohydrate, C14H28O6.H2O, is monoclinic, C2, with Z = 4, a = 17.896(2), b = 5.154(1), c = 18.303(2) A, beta = 90.30(1) degrees. The hemihydrate, C14H28O6.0.5 H2O, is also monoclinic, C2, with Z =
Brown, G.M., et al.
Canadian Journal of Chemistry, 48, 2525-2525 (1970)
Chaoqun Yao et al.
Proteomics. Clinical applications, 4(1), 4-16 (2010-12-08)
About two million new cases of leishmaniasis with 50 000 associated deaths occur worldwide each year. Promastigotes of the causative Leishmania spp. develop from the procyclic stage to the highly virulent metacyclic stage within the sand fly vector. We hypothesized
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service