G7502
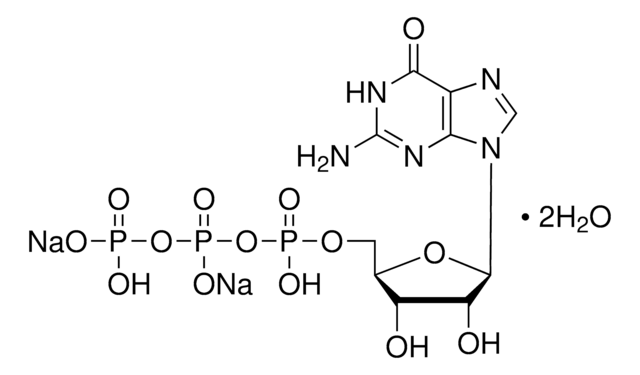
Guanosine 5′-diphosphoglucose sodium salt
Synonym(s):
GDP-Glc, GDP-Glucose, GDPG
About This Item
Recommended Products
storage temp.
−20°C
SMILES string
[Na].NC1=NC(=O)c2ncn(C3OC(COP(O)(=O)OP(O)(=O)OC4OC(CO)C(O)C(O)C4O)C(O)C3O)c2N1
InChI
1S/C16H25N5O16P2.Na.H/c17-16-19-12-6(13(28)20-16)18-3-21(12)14-10(26)8(24)5(34-14)2-33-38(29,30)37-39(31,32)36-15-11(27)9(25)7(23)4(1-22)35-15;;/h3-5,7-11,14-15,22-27H,1-2H2,(H,29,30)(H,31,32)(H3,17,19,20,28);;
InChI key
MEXITZOHWLXZKR-UHFFFAOYSA-N
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Glycosyltransferases were initially considered to be specific for a single glycosyl donor and acceptor, which led to the one enzyme-one linkage concept. Subsequent observations have refuted the theory of absolute enzymatic specificity by describing the transfer of analogs of some nucleoside mono- or diphosphate sugar donors.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service