D6783
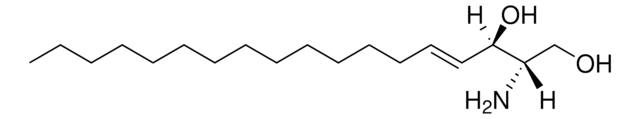
DL-Dihydrosphingosine
≥98%, synthetic
Synonym(s):
1,3-Dihydroxy-2-aminooctadecane, DL-1,3-Dihydroxy-2-aminooctadecane
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C18H39NO2
CAS Number:
Molecular Weight:
301.51
EC Number:
MDL number:
UNSPSC Code:
12352211
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
biological source
synthetic
Assay
≥98%
form
solid
storage temp.
−20°C
SMILES string
CCCCCCCCCCCCCCCC(O)C(N)CO
InChI
1S/C18H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(21)17(19)16-20/h17-18,20-21H,2-16,19H2,1H3
InChI key
OTKJDMGTUTTYMP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Dihydrosphingosine is a crucial constituent of the skin lipid barrier, which guards the body from excessive water loss.
Application
DL-Dihydrosphingosine has been used to initiate heat stress signals in Saccharomyces cerevisiae.
Biochem/physiol Actions
Biosynthetic precursor of sphingosine.
Other Notes
Mixture of erythro and threo isomers. Content given on label.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sphingolipids Are Potential Heat Stress Signals inSaccharomyces
Dickson RC, et al.
The Journal of Biological Chemistry, 272(48), 30196-30200 (1997)
Phytosphingosine, sphingosine and dihydrosphingosine ceramides in model skin lipid membranes: permeability and biophysics
vSkolova B, et al.
Biochimica et Biophysica Acta - Biomembranes, 1859(5), 824-834 (2017)
A Kawamura et al.
Bioorganic & medicinal chemistry, 4(7), 1035-1043 (1996-07-01)
We have developed a simple picomole (low nanogram) scale HPLC scheme which can separate all eight isomers of sphingosine and dihydrosphingosine thus leading to the identification of their relative and absolute configurations. The amino group of the sample is derivatized
E M Raeder et al.
Blood, 93(2), 686-693 (1999-01-13)
In the present study, we investigated the mechanism by which sphingosine and its analogues, dihydrosphingosine and phytosphingosine, inhibit polymorphonuclear leukocyte (PMN) phagocytosis of IgG-opsonized erythrocytes (EIgG) and inhibit ERK1 and ERK2 phosphorylation. We used antibodies that recognized the phosphorylated forms
Lucila Gisele Pescio et al.
Journal of lipid research, 58(7), 1428-1438 (2017-05-19)
Ceramides (Cers) and complex sphingolipids with defined acyl chain lengths play important roles in numerous cell processes. Six Cer synthase (CerS) isoenzymes (CerS1-6) are the key enzymes responsible for the production of the diversity of molecular species. In this study
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service