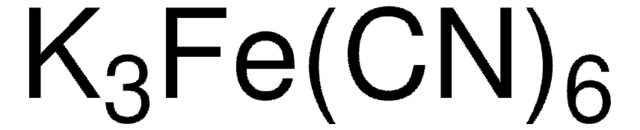P8131
Potassium hexacyanoferrate(III)
ReagentPlus®, ~99%
Synonym(s):
Potassium ferricyanide, Red prussiate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
K3Fe(CN)6
CAS Number:
Molecular Weight:
329.24
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.21
Assay:
~99%
form:
powder or crystals
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
~99%
form
powder or crystals
pH
6-9 (25 °C, 329 g/L)
SMILES string
[K+].[K+].[K+].N#C[Fe-3](C#N)(C#N)(C#N)(C#N)C#N
InChI
1S/6CN.Fe.3K/c6*1-2;;;;/q;;;;;;-3;3*+1
InChI key
MIMJFNVDBPUTPB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Potassium hexacyanoferrate(III) can be used as a one-electron oxidant for the oxidation of quinolines, thiamines, and cyclic amines.
Application
- Enzymatic synthesis of a skin active ingredient - glochidone by 3-ketosteroid dehydrogenase from Sterolibacterium denitrificans: Highlighting the utilization of Potassium hexacyanoferrate(III) in biochemical applications, specifically in enzymatic reactions involving steroid conversion processes (Wojtkiewicz AM et al., 2024).
- ZIFs-Derived Hollow Nanostructures via a Strong/Weak Coetching Strategy for Long-Life Rechargeable Zn-Air Batteries: Demonstrates the role of Potassium hexacyanoferrate(III) in creating nanostructured materials for enhanced electrochemical energy storage, pertinent to battery technology (Li S et al., 2024).
- Development of an electrochemical impedance spectroscopy immunosensor for insulin monitoring employing pyrroloquinoline quinone as an ingestible redox probe: Incorporates Potassium hexacyanoferrate(III) for developing advanced biosensors with applications in healthcare diagnostics (Khanwalker M et al., 2024).
- Selective gold extraction from electronic waste using high-temperature-synthesized reagents: This study uses Potassium hexacyanoferrate(III) in novel methodologies for the recovery of precious metals from electronic waste, offering a sustainable approach to waste management and recycling (Li J et al., 2024).
- Tunable Electrochemical Entropy through Solvent Ordering by a Supramolecular Host: Explores the chemical versatility of Potassium hexacyanoferrate(III) in creating responsive molecular environments for energy systems, underlining its importance in materials science and thermodynamic studies (Xia KT et al., 2023).
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2
Supplementary Hazards
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Simultaneous detection of thiamine and its phosphate esters from microalgae by HPLC
Pinto E, et al.
Biochemical and Biophysical Research Communications, 291, 344-348 (2002)
Osmium (VIII)-catalyzed oxidation of some cyclic amines by potassium hexacyanoferrate (III) in alkaline media: A kinetics and mechanistic study
Al-Subu M, et al.
Chemistry of Heterocyclic Compounds, 39, 478-484 (2003)
Behaviour of cooxidation of isopropyl ether of vitamin A with cumene in chlorobenzene
VaRdanyana R, et al.
Oxidation Communications, 36, 845-851 (2013)
Kinetics of Oxidation of Some Fluoroquinolones by Hexacyanoferrate (III) in Alkaline Medium
Diab N, et al.
International journal of chemistry research, 34, 1388-1388 (2013)
Catherine M Czeisler et al.
The Journal of physiology, 597(8), 2225-2251 (2019-02-02)
The embryonic PHOX2B-progenitor domain generates neuronal and glial cells which together are involved in chemosensory control of breathing and sleep homeostasis. Ablating PHOX2B-derived astrocytes significantly contributes to secondary hypoxic respiratory depression as well as abnormalities in sleep homeostasis. PHOX2B-derived astrocyte
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service