239283
Potassium hydrogenfluoride
99%
Synonym(s):
Potassium bifluoride, Potassium hydrogen difluoride
About This Item
powder or crystals
Recommended Products
grade
for analytical purposes
Assay
99%
form
lumps
powder or crystals
mp
239 °C (lit.)
density
2.37 g/mL at 25 °C (lit.)
SMILES string
[F-].[K+].F[H]
InChI
1S/2FH.K/h2*1H;/q;;+1/p-1
InChI key
VBKNTGMWIPUCRF-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
- Synthesis of 4′-deoxy-4′-fluorokanamycin A and B: This study discusses the synthetic applications of Potassium hydrogenfluoride in the development of fluorinated antibiotics, highlighting its role in selective fluorination processes (Takahashi Y et al., 1992).
- Reaction of 2-deoxy-6-O-[2,3-dideoxy-4,6-O-isopropylidene-2,3-(N-tosylepimino)-alpha-D-mannopyranosyl]-4,5-O-isopropylidene-1,3-di-N-tosylstreptamine with potassium hydrogenfluoride: This article explores the chemical behavior of Potassium hydrogenfluoride under specific synthetic conditions, providing insights into its role in complex organic transformations (Kobayashi Y et al., 1992).
- A synthetic study of methyl 3-deoxy-3-fluoro-alpha-D-glucopyranosides from methyl 2,3-anhydro-alpha-D-allopyranosides, and synthesis of 3′-deoxy-3′-fluorokanamycin A and 3′-chloro-3′-deoxykanamycin A: This research delves into the synthesis of modified sugars using Potassium hydrogenfluoride, illustrating its utility in the creation of novel glycoside structures, which are important in drug development (Umemura E et al., 1992).
Signal Word
Danger
Hazard Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service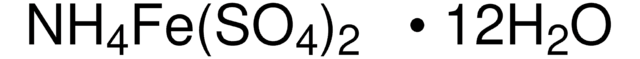









![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
