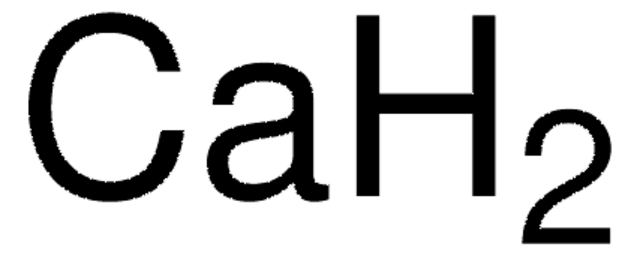208027
Calcium hydride
reagent grade, 95% (gas-volumetric)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
H2Ca
CAS Number:
Molecular Weight:
42.09
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
grade
reagent grade
Quality Level
Assay
95% (gas-volumetric)
form
powder, chunks or granules
reaction suitability
reagent type: reductant
mp
816 °C (lit.)
SMILES string
[Ca]
InChI
1S/Ca.2H
InChI key
FAQLAUHZSGTTLN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Calcium hydride shows promising abilities as a hydrogen storage system for alkaline fuel cells.
Application
Calcium hydride has been used as a drying agent for organic solvents.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Water-react 1
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Development of hydrogen storage for fuel cell generators II: utilization of calcium hydride and lithium hydride.
Kong VCY, et al.
International Journal of Hydrogen Energy, 28(2), 205-214 (2003)
Synthesis of 1,2-Azaborines and the Preparation of Their Protein Complexes with T4 Lysozyme Mutants.
Lee H and Liu S-Y.
Journal of Visualized Experiments, 121, e55154-e55154 (2017)
Lucas A Kinard et al.
Nature protocols, 7(6), 1219-1227 (2012-06-02)
This protocol describes the synthesis of oligo(poly(ethylene glycol) fumarate) (OPF; 1-35 kDa; a polymer useful for tissue engineering applications) by a one-pot reaction of poly(ethylene glycol) (PEG) and fumaryl chloride. The procedure involves three parts: dichloromethane and PEG are first
Robert Weinmeister et al.
ACS nano, 9(10), 9718-9730 (2015-09-15)
Aqueous microdroplets with a volume of a few femtoliters are an ideal sample size for single-molecule fluorescence experiments. In particular, they enable prolonged measurements to be made on individual molecules that can diffuse freely in the surrounding medium. However, the
Yin-Chih Fu et al.
Acta biomaterialia, 10(11), 4583-4596 (2014-07-23)
Nanoparticles (NP) that target bone tissue were developed using PLGA-PEG (poly(lactic-co-glycolic acid)-polyethylene glycol) diblock copolymers and bone-targeting moieties based on aspartic acid, (Asp)(n(1,3)). These NP are expected to enable the transport of hydrophobic drugs. The molecular structures were examined by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service