1.08262
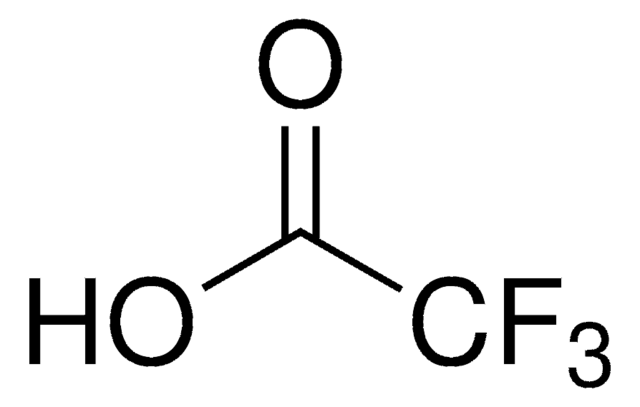
Trifluoroacetic acid
for spectroscopy Uvasol®
Synonym(s):
TFA
About This Item
Recommended Products
vapor density
3.9 (vs air)
Quality Level
vapor pressure
97.5 mmHg ( 20 °C)
Assay
≥99.8% (acidimetric)
form
liquid
technique(s)
UV/Vis spectroscopy: suitable
impurities
≤10% Water (Karl Fischer)
evapn. residue
≤0.005%
color
APHA: ≤10
transmittance
265 nm, ≥10.0%
305 nm, ≥50.0%
320 nm, ≥80.0%
325 nm, ≥90.0%
refractive index
n20/D 1.3 (lit.)
pH
1 (10 g/L in H2O)
bp
72.4 °C (lit.)
mp
−15.4 °C (lit.)
solubility
soluble 10 g/mL
density
1.489 g/mL at 20 °C (lit.)
storage temp.
2-30°C
SMILES string
OC(C(F)(F)F)=O
InChI
1S/C2HF3O2/c3-2(4,5)1(6)7/h(H,6,7)
InChI key
DTQVDTLACAAQTR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Characterization of ribostamycin and its impurities using a nano-quantity analyte detector: This study examines ribostamycin and its impurities using a nano-quantity analyte detector and details a systematic comparison of three different aerosol detectors. The role of trifluoroacetic acid as a mobile phase additive enhances detection sensitivity and analytical precision, making it crucial for accurate impurity profiling and pharmaceutical quality control (Meng et al., 2024).
- Paradigm Shift: Major Role of Ion-Pairing-Dependent Size Exclusion Effects in Bottom-Up Proteomics Reversed-Phase Peptide Separations: This research highlights the critical role of trifluoroacetic acid in modifying the ion-pairing and size exclusion properties of peptides during reversed-phase separations in proteomics. The study provides a deeper understanding of the mechanisms influencing peptide behavior, which is essential for developing more effective analytical techniques (Yeung et al., 2024).
- New fabric phase sorptive extraction for nondestructive analysis of heritage textile samples: This paper introduces a novel fabric phase sorptive extraction method using trifluoroacetic acid among other solvents to analyze heritage textile samples nondestructively. This method demonstrates significant potential in cultural heritage preservation and forensic science by allowing detailed analysis without damaging the original artifacts (Tanasescu et al., 2024).
- Preparation and characterization of stationary phase gradients on C8 liquid chromatography columns: The study discusses the preparation and characterization of stationary phase gradients for chromatography columns, with trifluoroacetic acid used to adjust the pH and ionic strength of the mobile phase. This modification is shown to enhance the chromatographic separation of complex mixtures, proving vital for analytical and preparative scale separations (Cecil et al., 2024).
- Optimization of Cyanocobalamin (Vitamin B(12)) Sorption onto Mesoporous Superparamagnetic Iron Oxide Nanoparticles: This investigation utilizes trifluoroacetic acid in the synthesis and functionalization of nanoparticles for efficient vitamin B(12) sorption. The study explores the implications for targeted drug delivery systems and bioseparation, demonstrating the adaptability and effectiveness of these engineered nanoparticles (Flieger et al., 2024).
Analysis Note
Water (K. F.): ≤ 0.10 %
Colour number (Apha): ≤ 10
Evaporation residue: ≤ 0.005 %
UV-transmission (at 265 nm): ≥ 10.0 %
UV-transmission (at 305 nm): ≥ 50.0 %
UV-transmission (at 320 nm): ≥ 80.0 %
UV-transmission (from 325 nm): ≥ 90.0 %
Other Notes
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1A
Storage Class Code
8A - Combustible, corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
212.0 °F - Pensky-Martens closed cup
Flash Point(C)
> 100 °C - Pensky-Martens closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Being one of the most popular flavors, we present a HPLC fingerprint method and vanilla extract reference materials to help distinguish natural from synthetic or adulterated vanilla.
Protocols
Straightforward HPTLC-MS analysis of lactose in dairy products (milk or yoghurt) using only protein crash, centrifugation and dilution as sample preparation.
Aripiprazole is an atypical antipsychotic and a partial dopamine agonist. It is primarily used in the treatment of schizophrenia, bipolar disorder, major depressive disorder, tic disorders, and irritability associated with autism. Aripiprazole was first approved by the U.S. Food and DrugAdministration in November 2002 for schizophrenia and by the European Medicines Agency in June 2004 for acute manic and mixed episodes associated with bipolar disorder.
Catalyst screening, KitAlysis™ High-Throughput Screening Kits, Thin layer chromatography (TLC), parallel analysis, parallel synthesisigh-Throughput Buchwald-Hartwig Amination Reaction Screening Kit, TLC-MS analysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service