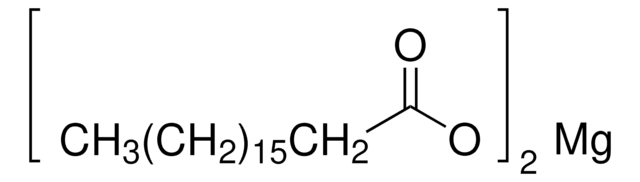
1.04429
Parteck® LM low
EMPROVE® ESSENTIAL
Pharma Manufacturing
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352107
Recommended Products
grade
GMO free
GMP
Quality Level
description
granulated
product line
EMPROVE® ESSENTIAL
form
granular
particle size
80-220 μm (d50)
application(s)
pharmaceutical
solid formulation
storage temp.
2-25°C
General description
With formulation challenges in mind, we have used particle engineering technologies to develop functional excipients specifically for solid oral dosage forms. The products in our Parteck® portfolio feature unique particle properties tailored for excellent performance in tableting processes, for specific drug delivery technologies, or for solubility enhancement.
As part of our Emprove® Program, our raw materials are offered with extensive documentation facilitating compliance of your pharma and biopharma product, full supply chain transparency and risk mitigation. Our SAFC® portfolio of high-quality products for biopharmaceutical and pharmaceutical formulation and production withstands strict quality control procedures and is produced according to applicable cGMP guidelines.
As part of our Emprove® Program, our raw materials are offered with extensive documentation facilitating compliance of your pharma and biopharma product, full supply chain transparency and risk mitigation. Our SAFC® portfolio of high-quality products for biopharmaceutical and pharmaceutical formulation and production withstands strict quality control procedures and is produced according to applicable cGMP guidelines.
Application
Parteck® LM excipient comes with three functions: filler, binder, and lubricant for solid dose formulation. It shows excellent flow properties and allows consistent dosing and robust, constant mixing times in batch and continuous manufacturing processes. It is very well suited for all classical solid dose formulation techniques such as direct compression, roller compaction, dry granulation as well as capsule filling.
Two grades are available: Parteck® LM low for low dose applications and less requirement for lubrication. Parteck® LM high for higher API loads and more demanding cases with regards to lubrication.
Two grades are available: Parteck® LM low for low dose applications and less requirement for lubrication. Parteck® LM high for higher API loads and more demanding cases with regards to lubrication.
Features and Benefits
- Multifunctional excipient for compression tableting processes – Filler + binder + lubricant
- Reduces dosing issues in continuous manufacturing
- Handling robustness supports limitation of overlubrication in batch operation
- High binding capacity = strong tablets
- Proven lubrication efficiency
- Two grades allow for flexibility in formulation development
Packaging
1.04429.1000 / 1 kg / PE bottle
1.04429.9025 / 25 kg / PE bag (in carton)
1.04429.9025 / 25 kg / PE bag (in carton)
Legal Information
Emprove is a registered trademark of Merck KGaA, Darmstadt, Germany
PARTECK is a registered trademark of Merck KGaA, Darmstadt, Germany
SAFC is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service