All Photos(1)
About This Item
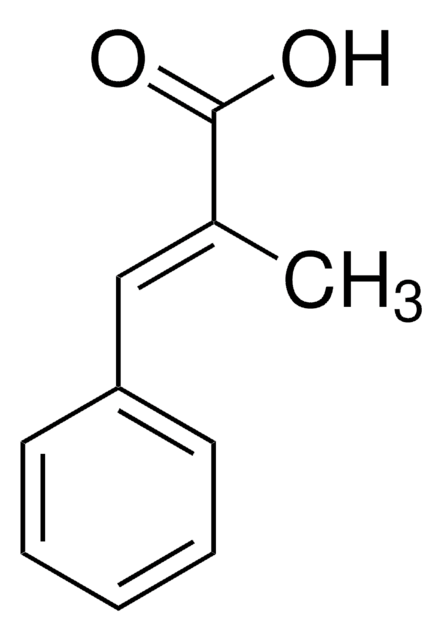
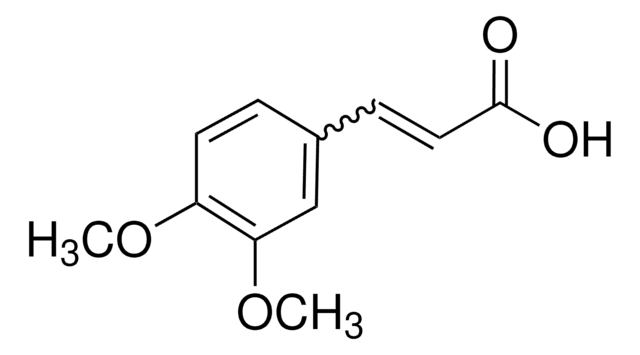
Linear Formula:
(CH3O)3C6H2CH=CHCO2H
CAS Number:
Molecular Weight:
238.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
97%
mp
125-127 °C (lit.)
SMILES string
COc1cc(\C=C\C(O)=O)cc(OC)c1OC
InChI
1S/C12H14O5/c1-15-9-6-8(4-5-11(13)14)7-10(16-2)12(9)17-3/h4-7H,1-3H3,(H,13,14)/b5-4+
InChI key
YTFVRYKNXDADBI-SNAWJCMRSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yingming Wu et al.
Organic letters, 11(3), 597-600 (2008-12-25)
A highly flexible and concise total synthesis of (+)-podophyllotoxin featured with an enantioselective sequential conjugate addition-allylation reaction was reported. Starting from commercially available 3,4,5-trimethoxycinnamic acid, this new route leads to (+)-podophyllotoxin 1 in only eight steps with 29% overall yield.
Keiko Kawashima et al.
Biological & pharmaceutical bulletin, 27(8), 1317-1319 (2004-08-12)
3,4,5-trimethoxycinnamic acid (TMCA) is one of the constituents in Onji (roots of Polygala tenuifolia WILLD), an herbal medicine used for sedative in Japanese traditional Kampo medicine. Our previous study revealed that oral administration of this compound prolongs sleeping time induced
M I Donnelly et al.
Journal of bacteriology, 147(2), 471-476 (1981-08-01)
When grown on 3,4,5-trimethoxycinnamic acid, a strain of Pseudomonas putida oxidized this compound and also 3,4,5-trimethoxybenzoic, 3,5-dimethoxy-4-hydroxybenzoic (syringic), and 3,4-dihydroxy-5-methoxybenzoic (3-O-methylgallic) acids, but 3,5-dimethoxy-4-hydroxycinnamic and other acids bearing structural resemblances to the growth substrate were oxidized only slowly. These results
Jae-Chul Jung et al.
Chemical biology & drug design, 81(3), 389-398 (2012-11-06)
A series of 3,4,5-trimethoxycinnamic acid derivatives was prepared and evaluated for antinarcotic effects on morphine dependence in mice and binding affinities on serotonergic receptors. The key synthetic strategies involve generation of ketones 6-7, esters 9-12 through condensation reaction, and amides
Sarvesh Kumar et al.
Biochemistry, 44(48), 15944-15952 (2005-11-30)
We report here the isolation and characterization of two active principles, ethyl 3',4',5'-trimethoxycinnamate (1) and piperine (2), from the combined hexane and chloroform extracts of Piper longum. Using primary human umbilical vein endothelial cells, we evaluated the activities of compound
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service