P23989
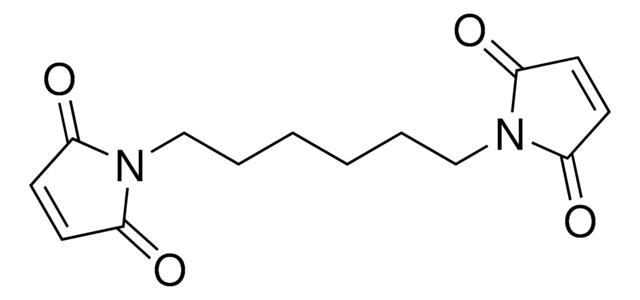
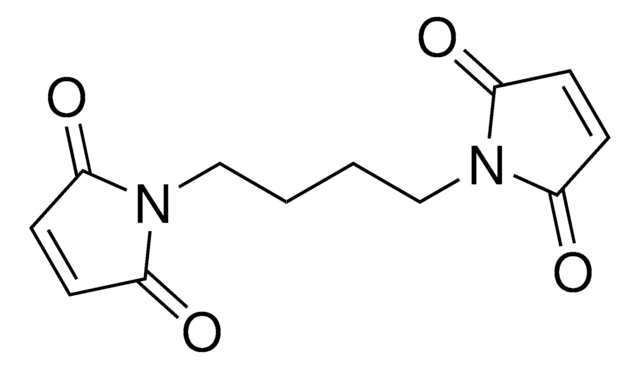
N,N′-(1,4-Phenylene)dimaleimide
97%
Synonym(s):
1,4-Dimaleimidobenzene, N,N′-(p-Phenylene)dimaleimide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H8N2O4
CAS Number:
Molecular Weight:
268.22
Beilstein:
249631
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
Recommended Products
Assay
97%
form
powder
mp
>300 °C (lit.)
SMILES string
O=C1C=CC(=O)N1c2ccc(cc2)N3C(=O)C=CC3=O
InChI
1S/C14H8N2O4/c17-11-5-6-12(18)15(11)9-1-2-10(4-3-9)16-13(19)7-8-14(16)20/h1-8H
InChI key
AQGZJQNZNONGKY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ryan N Mello et al.
Biophysical journal, 102(5), 1088-1096 (2012-03-13)
We have used thiol cross-linking and electron paramagnetic resonance (EPR) to resolve structural transitions of myosin's light chain domain (LCD) and catalytic domain (CD) that are associated with force generation. Spin labels were incorporated into the LCD of muscle fibers
K Polosukhina et al.
Biochemistry, 36(39), 11952-11958 (1997-10-08)
Rate constants for the reactions of Cys-697 and Cys-707 of skeletal muscle myosin subfragment 1 (S1) with N,N'-p-phenylenedimaleimide (pPDM) and its monofunctional analog phenylmaleimide (PM) were measured for S1 and S1 bound to nucleotides and/or actin. The [pPDM] and [PM]
M O Steinmetz et al.
The Journal of cell biology, 138(3), 559-574 (1997-08-11)
The effect of the type of metal ion (i.e., Ca2+, Mg2+, or none) bound to the high-affinity divalent cation binding site (HAS) of actin on filament assembly, structure, and dynamics was investigated in the absence and presence of the mushroom
Yu S Borovikov et al.
Biophysical journal, 86(5), 3020-3029 (2004-04-28)
Fluorescence polarization measurements were used to study changes in the orientation and order of different sites on actin monomers within muscle thin filaments during weak or strong binding states with myosin subfragment-1. Ghost muscle fibers were supplemented with actin monomers
Q Wang et al.
Journal of molecular biology, 291(3), 683-692 (1999-08-17)
The lactose permease of Escherichia coli was expressed in two fragments (split permease), each with a Cys residue, and cross-linking was studied. Split permease with a discontinuity in either loop II/III (N2C10permease) or loop VI/VII (N6C6permease) was used. Proximity of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service