C100005
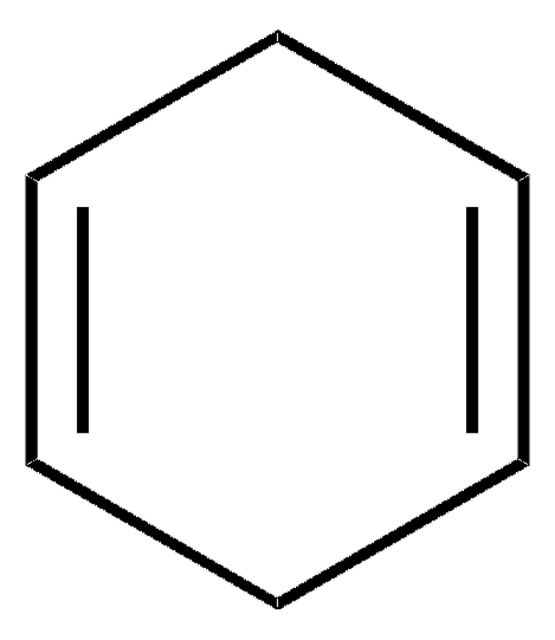
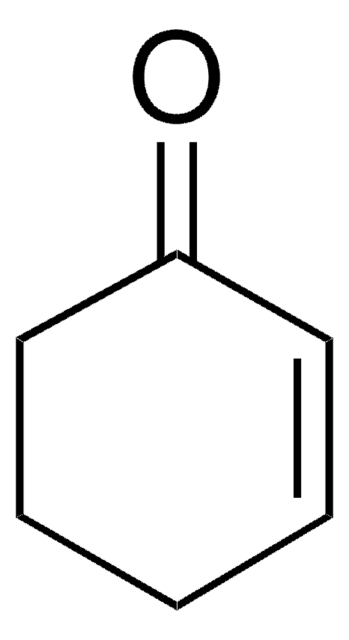
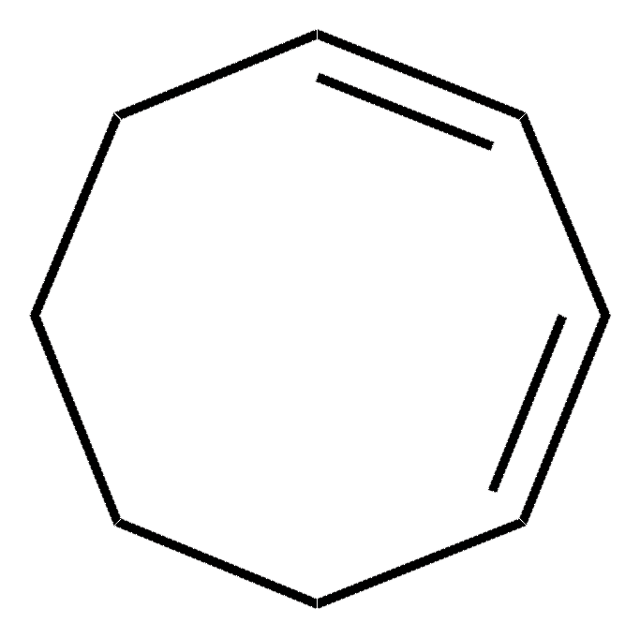
1,3-Cyclohexadiene
contains 0.05% BHT as inhibitor, 97%
Synonym(s):
1,2-Dihydrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8
CAS Number:
Molecular Weight:
80.13
Beilstein:
506024
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
contains
0.05% BHT as inhibitor
refractive index
n20/D 1.474 (lit.)
bp
80 °C (lit.)
density
0.841 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C1CC=CC=C1
InChI
1S/C6H8/c1-2-4-6-5-3-1/h1-4H,5-6H2
InChI key
MGNZXYYWBUKAII-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1,3-Cyclohexadiene can undergo:
- C-C coupling with aromatic alcohols via iridium-catalyzed hydrogen auto-transfer and with aldehydes via transfer hydrogenation mediated by isopropanol to form carbonyl addition products.
- Living anionic polymerization with n-BuLi/TMEDA system to form polycyclohexadiene.
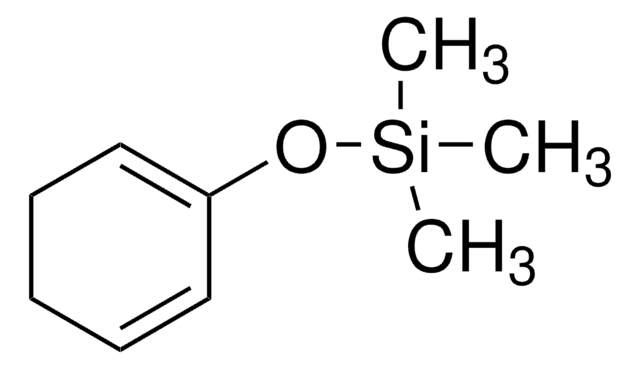
- Platinum-catalyzed silaboration to form (1R,4S)-1-(dimethylphenylsilyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-cyclohexene.
- Aerobic palladium-catalyzed 1,4-diacetoxylation in the presence of cobalt tetra(hydroquinone)porphyrin as an electron transfer reagent.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
66.0 °F
Flash Point(C)
18.9 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sarah J Ryan et al.
Journal of the American Chemical Society, 133(13), 4694-4697 (2011-03-12)
Herein we report the first all-carbon N-heterocyclic carbene-catalyzed (4 + 2) cycloaddition. The reaction proceeds with α,β-unsaturated acid fluorides and silyl dienol ethers and produces 1,3-cyclohexadienes with complete diastereocontrol (dr >20:1) while demonstrating a new type of reaction cascade exploiting
Contact formation dynamics: Mapping chemical bond formation between a molecule and a metallic probe.
Borislav Naydenov et al.
Nano letters, 6(8), 1752-1756 (2006-08-10)
We present a study that maps out chemical bond formation between a Pt-inked probe and a single 1,3-cyclohexadiene (1,3-CHD) molecule on Si(100). By separating the mechanical and electronic contributions to the current during the approach to contact, we show that
Enantioselective Platinum?Catalyzed Silicon?Boron Addition to 1, 3?Cyclohexadiene.
Gerdin M and Moberg C
Advanced Synthesis & Catalysis, 347(6), 749-753 (2005)
Marija Kotur et al.
The Journal of chemical physics, 130(13), 134311-134311 (2009-04-10)
We demonstrate the use of shaped ultrafast laser pulses in the deep ultraviolet to control the ring opening isomerization of 1,3-cyclohexadiene to form 1,3,5-hexatriene. The experiments are performed with a gas phase sample and the isomerization yield is probed with
Chemo-, regio-, and stereoselective cobalt-mediated [2+2+2] cycloaddition of alkynyl boronates to alkenes: 1,3- and 1,4-diboryl-1,3-cyclohexadienes.
Vincent Gandon et al.
Angewandte Chemie (International ed. in English), 44(43), 7114-7118 (2005-10-12)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service