901686
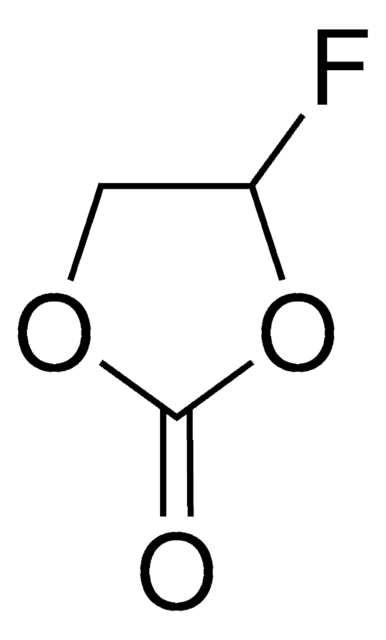
Fluoroethylene carbonate
battery grade, ≥99%, acid <200 ppm, anhydrous
Synonym(s):
4-Fluoro-1,3-dioxolan-2-one, FEC
About This Item
Recommended Products
grade
anhydrous
battery grade
Assay
≥99%
form
liquid
impurities
≤100 ppm H2O
≤200 ppm acid
bp
212 °C
mp
18-23 °C
density
1.485 g/cm3
application(s)
battery manufacturing
SMILES string
FC1COC(=O)O1
InChI
1S/C3H3FO3/c4-2-1-6-3(5)7-2/h2H,1H2
InChI key
SBLRHMKNNHXPHG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Features and Benefits
✔ Improves Battery Safety
✔ Enhances Battery Performance
✔ Versatile Electrode Compatibility
Caution
- These electrolyte solutions have extremely low water content; please handle under inert and moisture free environment (glove box).
- Keep containers tightly closed. Keep away from heat and ignition sources. Store in a cool and dry place. Avoid storing together with oxidizers.
Legal Information
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service