900258
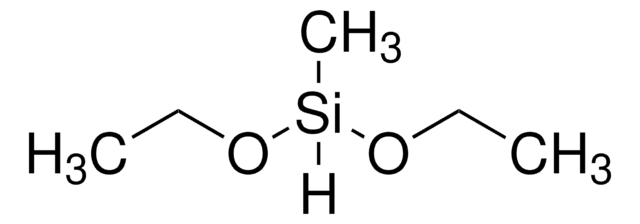
Isopropoxy(phenyl)silane
Synonym(s):
RubenSilane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H14OSi
CAS Number:
Molecular Weight:
166.29
UNSPSC Code:
12352005
NACRES:
NA.22
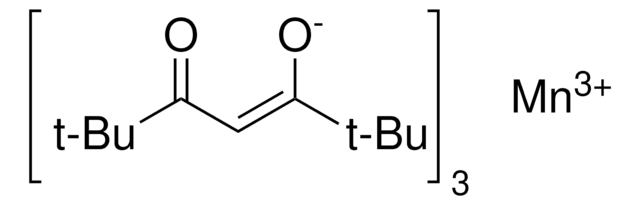
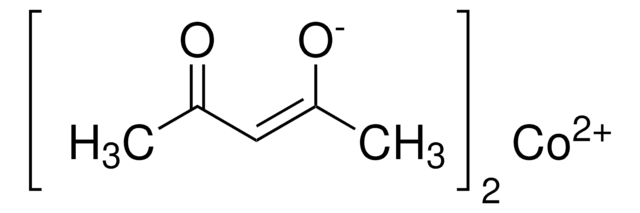
Recommended Products
form
liquid
Quality Level
reaction suitability
reagent type: reductant
reaction type: Reductions
refractive index
n/D 1.4799
density
0.926 g/mL
storage temp.
2-8°C
General description
Isopropoxy(phenyl)silane is used as a stoichiometric reductant that allows for a significant decrease in catalyst loading, lower reaction temperatures, a wide range of functional group tolerance, and more diverse solvents in metal-catalyzed Mukaiyama hydrofunctionalizations.
Application
Isopropoxy(phenyl)silane can be used as a hydride source for alkene hydrofunctionalization reactions in the presence of metal catalysts, particularly under aprotic, nonalcoholic conditions. This reagent is considered as one of the most efficient stoichiometric reductants than phenylsilane because of its ability to exclude alcohol solvent from a series of catalytic reactions. When utilized, it allows the researcher to significantly decrease catalyst loadings, lower reaction temperatures, and employ more diverse solvents in iron- and manganese-catalyzed Mukaiyama hydrofunctionalizations.
It can be used as a reagent in:
It can be used as a reagent in:
- The hydrogenation of olefins
- Branch-selective olefin cross-coupling reactions
- Markovnikov alkene hydration/amination reactions
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Carla Obradors et al.
Journal of the American Chemical Society, 138(14), 4962-4971 (2016-03-18)
We report the discovery of an outstanding reductant for metal-catalyzed radical hydrofunctionalization reactions. Observations of unexpected silane solvolysis distributions in the HAT-initiated hydrogenation of alkenes reveal that phenylsilane is not the kinetically preferred reductant in many of these transformations. Instead
Isopropoxy (phenyl) silane
Demoret RM and Shenvi RA
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service