852767
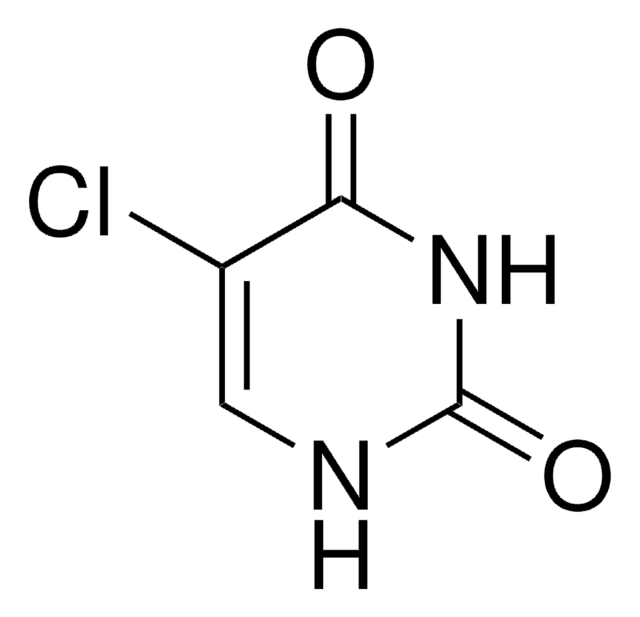
5-Nitrouracil
98%
Synonym(s):
2,4-Dihydroxy-5-nitropyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H3N3O4
CAS Number:
Molecular Weight:
157.08
Beilstein:
10410
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
form
powder
mp
>300 °C (lit.)
SMILES string
[O-][N+](=O)C1=CNC(=O)NC1=O
InChI
1S/C4H3N3O4/c8-3-2(7(10)11)1-5-4(9)6-3/h1H,(H2,5,6,8,9)
InChI key
TUARVSWVPPVUGS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Enzyme inhibitor.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T Kita et al.
Biological & pharmaceutical bulletin, 24(7), 860-862 (2001-07-18)
Several N-phenylhomophthalimide derivatives were prepared and their inhibitory activity on thymidine phosphorylase/ platelet-derived endothelial cell growth factor (TP/PD-ECGF) was assessed. Among them, 2-(2,6-diethylphenyl)-7-nitro-1,2,3,4-tetrahydroisoquinoline-1,3-dione (9) was found to be a more potent inhibitor than the classical inhibitor, 5-nitrouracil (1). Lineweaver-Burk plot
S G Stepan'ian et al.
Biofizika, 34(5), 753-757 (1989-09-01)
The high-resolution IR-spectra of 5-nitrouracil and 5-bromouracil isolated in Ar matrices at 11 K were obtained for the first time. The conformational structure of uracil 5-substituents--thymine, 5-bromouracil, 5-nitrouracil--is calculated by the molecular mechanics and quantum--chemical MINDO/3 methods. The possibility of
X Gu et al.
Nucleic acids research, 24(6), 1059-1064 (1996-03-15)
tRNA in which uracil is completely replaced by 5-nitro-uracil was prepared by substituting 5-nitro-UTP for UTP in an in vitro transcription reaction. The rationale was that the 5-nitro substituent activates the 6-carbon of the Ura heterocycle towards nucleophiles, and hence
P J Nykänen et al.
APMIS : acta pathologica, microbiologica, et immunologica Scandinavica, 96(9), 768-772 (1988-09-01)
The degradation of 3H-thymidine under various cell culture conditions was analysed. It was found that a half of 3H-thymidine was degraded to 3H-thymine during 24 hours in PHA stimulation of blood lymphocytes. A control culture in which PHA was not
F H Lin et al.
Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.), 189(3), 353-361 (1988-12-01)
The kinetics of conversion of 5-fluoro-2'-deoxyuridine (FdUrd) to 5-fluorouracil (FUra) by isolated rat intestinal epithelial cells was investigated. Also, the effects of potential inhibitors of this reaction, which is catalyzed by uridine phosphorylase and thymidine phosphorylase, were determined. A 2.5%
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service