808350
TokeOni
Synonym(s):
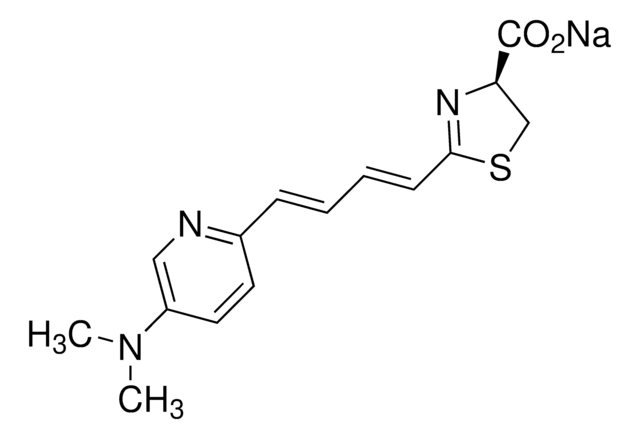
2-[(1E,3E)-4-[4-(Dimethylamino)phenyl]-1,3-butadien-1-yl]-4,5-dihydro-(4S)-4-thiazolecarboxylic acid hydrochloride salt, AkaLumine-HCl, Soluble NIR emission luciferin
About This Item
Recommended Products
form
powder
Quality Level
storage temp.
−20°C
SMILES string
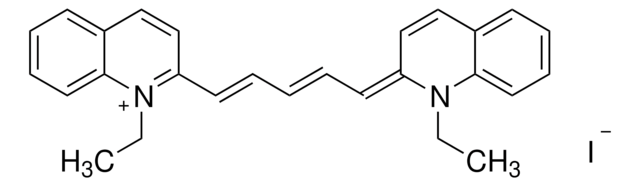
CN(C)C1=CC=C(/C=C/C=C/C2=N[C@@H](C(O)=O)CS2)C=C1.[H]Cl
InChI
1S/C16H18N2O2S.ClH/c1-18(2)13-9-7-12(8-10-13)5-3-4-6-15-17-14(11-21-15)16(19)20;/h3-10,14H,11H2,1-2H3,(H,19,20);1H/b5-3+,6-4+;/t14-;/m1./s1
InChI key
PZCNKVAGZCXXHX-SSRSOBHISA-N
General description
- Luciferin is a generic term for the light-emitting compound found in organisms that generate bioluminescence.
- TokeOni is a new NIR emission luciferin analogue having luminescence peak at 670 ~ 680 nm with a peak range in the near-infrared (NIR) region.
- TokeOni has the enhanced solubility of >10 mg/mL compared to HCl free (0.2 mg/mL).
Application
- Bioimaging.
- in vivo imaging.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Overall results suggest that seMpai is a suitable substrate of near-infrared BLI for many biological experiments. Its high solubility in neutral buffer conditions further extends the bioluminescent application of TokeOni derivatives.
Bioluminescence imaging (BLI) systems allows for high-sensitive and noninvasive monitoring of cell proliferation, activity of signaling pathways and protein-protein interactions in living tissues.
Synthesis and Applications of Graphene Nanoribbons Synthesized
Related Content
Organic electronics utilizes organic conductors and semiconductors for applications in organic photovoltaics, organic light-emitting diodes, and organic field-effect transistors.
Organic electronics utilizes organic conductors and semiconductors for applications in organic photovoltaics, organic light-emitting diodes, and organic field-effect transistors.
Organic electronics utilizes organic conductors and semiconductors for applications in organic photovoltaics, organic light-emitting diodes, and organic field-effect transistors.
Organic electronics utilizes organic conductors and semiconductors for applications in organic photovoltaics, organic light-emitting diodes, and organic field-effect transistors.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service