795291
Phenofluor™ solution
0.1 M in toluene
Synonym(s):
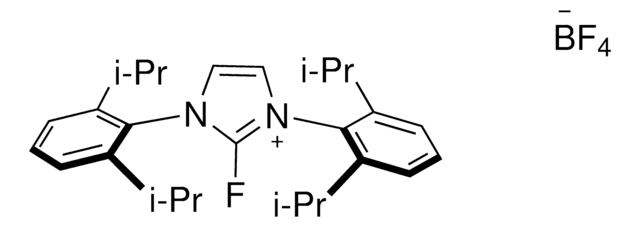
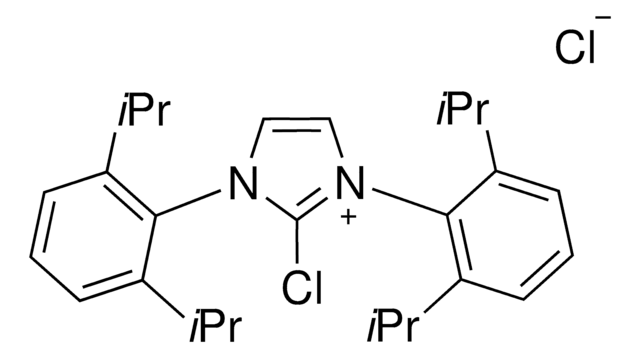
1,3-Bis(2,6-diisopropylphenyl)-2,2-difluoro-4-imidazoline
About This Item
Recommended Products
form
liquid
Quality Level
concentration
0.1 M in toluene
density
0.865 g/mL at 25 °C
storage temp.
2-8°C
InChI
1S/C27H36F2N2/c1-17(2)21-11-9-12-22(18(3)4)25(21)30-15-16-31(27(30,28)29)26-23(19(5)6)13-10-14-24(26)20(7)8/h9-20H,1-8H3
InChI key
FQXXWTOSPDVNSG-UHFFFAOYSA-N
Application
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
39.2 °F
Flash Point(C)
4 °C
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The functional-group-tolerant, one-step conversion of phenols to aryl fluorides is a highly desirable process. PhenoFluor™ solution, provides straightforward method to fluorinate alcohol and phenols without preactivation and regioselectively fluorinate highly functionalized late-stage intermediates.
Related Content
The Ritter lab currently focuses on fluorination chemistry for late-stage functionalization of complex natural and unnatural products. PhenoFluor™ has been developed as a general reagent for the selective, predictable, direct deoxyfluorination of complex alcohols and phenols.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)


