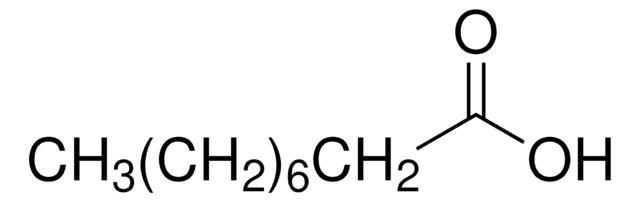75940
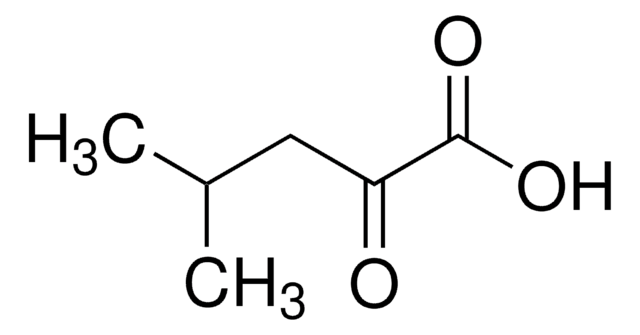
2-Oxooctanoic acid
≥99.0% (T)
Synonym(s):
α-Ketooctanoic acid, 2-Oxocaprylic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
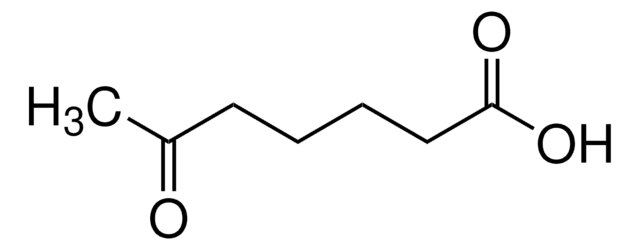
CH3(CH2)5COCOOH
CAS Number:
Molecular Weight:
158.19
Beilstein:
1757862
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99.0% (T)
form
flakes
bp
82 °C/0.05 mmHg
mp
33-36 °C
functional group
carboxylic acid
storage temp.
2-8°C
SMILES string
CCCCCCC(=O)C(O)=O
InChI
1S/C8H14O3/c1-2-3-4-5-6-7(9)8(10)11/h2-6H2,1H3,(H,10,11)
InChI key
GPPUPQFYDYLTIY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Huifang Xu et al.
Journal of colloid and interface science, 509, 265-274 (2017-09-16)
Single-tailed short-chain alkyl keto-acids/salts, a class of fatty acid/salt derivatives, such as sodium 2-ketooctanate (KOCOONa), are a kind of weakly acid/salt type amphiphiles and plausible prebiotic molecules, and the current understanding of their aggregation behavior in aqueous solutions is still
Sarah A Almahboub et al.
Applied microbiology and biotechnology, 102(2), 789-799 (2017-11-28)
Terminal modification of peptides is frequently used to improve their hydrophobicity. While N-terminal modification with fatty acids (lipidation) has been reported previously, C-terminal lipidation is limited as it requires the use of linkers. Here we report the use of a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service