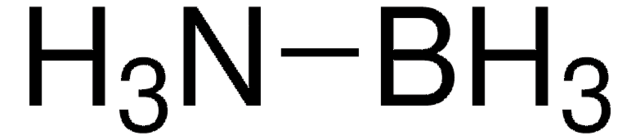682098
Borane-ammonia complex
95%
Synonym(s):
Amminetrihydroboron, Borazane
About This Item
Recommended Products
Assay
95%
form
solid
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
greener alternative category
SMILES string
B.N
InChI
1S/BH3.H3N/h2*1H3
InChI key
WZMUUWMLOCZETI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service