All Photos(1)
About This Item
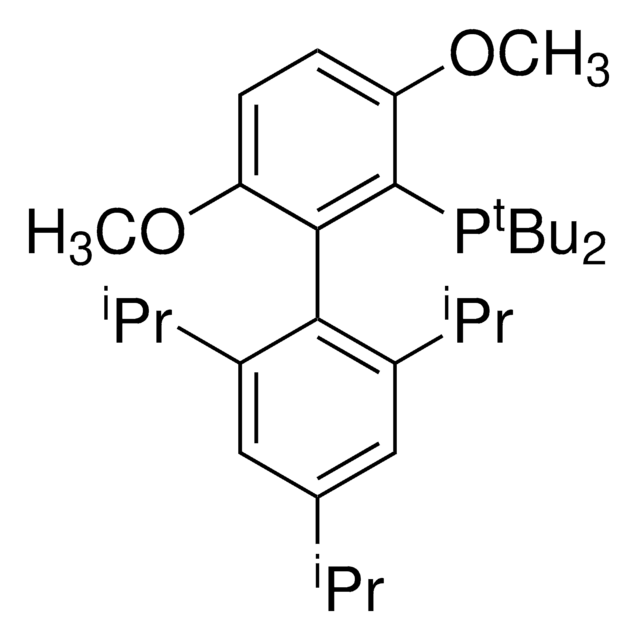
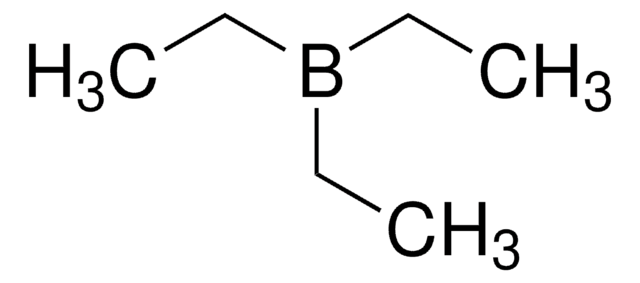
Linear Formula:
(C2H5)3B
CAS Number:
Molecular Weight:
97.99
Beilstein:
1731462
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
Recommended Products
reaction suitability
reagent type: reductant
concentration
2.0 M in diethyl ether
density
0.703 g/mL at 25 °C
SMILES string
CCB(CC)CC
InChI
1S/C6H15B/c1-4-7(5-2)6-3/h4-6H2,1-3H3
InChI key
LALRXNPLTWZJIJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Catalyst for:
Reactant for radical reductions of alkyl bromides with N-heterocyclic carbene boranes
Reactant for synthesis of tetramethylammonium trialkylphenylborate salts with oxidation potential
- Allylation of aldehydes
- Decarboxylative C-C bond cleavage reactions
- Rhenium hydride / boron Lewis acid cocatalysis of alkene hydrogenations
- Regioselective hydroxyalkylation of unsaturated oxime ethers
Reactant for radical reductions of alkyl bromides with N-heterocyclic carbene boranes
Reactant for synthesis of tetramethylammonium trialkylphenylborate salts with oxidation potential
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1A - STOT SE 3
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Flash Point(F)
-40.0 °F
Flash Point(C)
-40 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hideto Miyabe et al.
Chemical & pharmaceutical bulletin, 51(5), 540-544 (2003-05-09)
Stereocontrol in radical reactions of oxime ether anchored to polymer support was studied. Highly diastereoselective solid-phase radical reaction was achieved by using triethylborane and diethylzinc as a radical initiator at low reaction temperature, providing a novel method for the synthesis
Masafumi Ueda
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 124(6), 311-319 (2004-06-01)
The aqueous medium radical reactions of a variety of imine derivatives such as oxime ether, oxime, hydrazone, nitrone, and N-sulfonylimine were investigated. Triethylborane-mediated intermolecular alkyl radical addition to glyoxylic oxime ether, oxime, and nitrone in water proceeded smoothly to give
Ken-ichi Yamada et al.
The Journal of organic chemistry, 77(3), 1547-1553 (2012-01-03)
Triethylborane-mediated tin-free radical alkylation of N-alkoxycarbonyl-imines, such as N-Boc-, N-Cbz-, and N-Teoc-imines, proceeded smoothly at a low temperature (-78 to -20 °C) to give the corresponding adducts in high yield. Although the formation of isocyanate was the major unfavorable reaction
Hideto Miyabe et al.
Chemical & pharmaceutical bulletin, 52(1), 74-78 (2004-01-08)
Stannyl radical addition-cyclization of oxime ethers connected with olefin moieties was studied. The radical reactions proceeded effectively by the use of triethylborane as a radical initiator to provide the functionalized pyrrolidines via a carbon-carbon bond-forming process.
Hideto Miyabe
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 123(5), 285-294 (2003-05-30)
This review summarizes the new carbon-carbon bond construction methods based on the radical reaction of imine derivatives. The intermolecular carbon radical addition to oxime ethers proceeded smoothly in the presence of BF3.OEt2. A high degree of stereocontrol in the reaction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service