576638
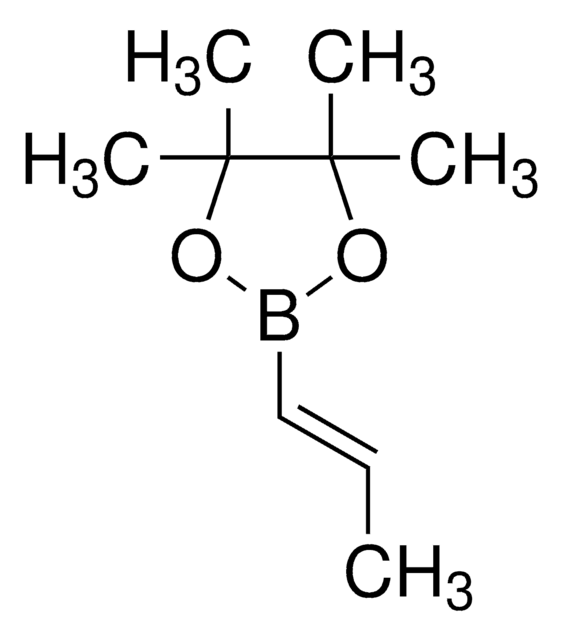
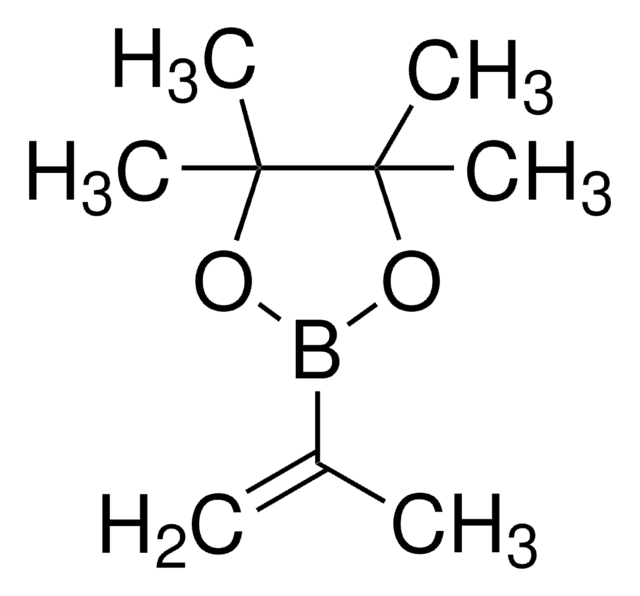
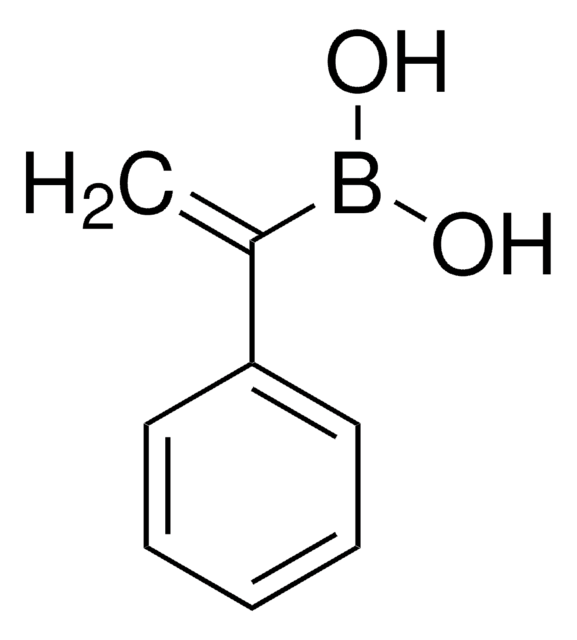
trans-1-Propen-1-ylboronic acid
≥95.0%
Synonym(s):
(E)-1-Propen-1-ylboronic acid, (E)-Prop-1-enylboronic acid, trans-1-Propeneboronic acid, trans-1-Propenylboronic acid, trans-Propenylboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3CH=CHB(OH)2
CAS Number:
Molecular Weight:
85.90
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
impurities
~10 wt. % cis-isomer
mp
123-127 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]\C(C)=C(\[H])B(O)O
InChI
1S/C3H7BO2/c1-2-3-4(5)6/h2-3,5-6H,1H3/b3-2+
InChI key
CBMCZKMIOZYAHS-NSCUHMNNSA-N
Related Categories
Application
Reactant for:
Reactant for preparation of:
- Palladium-phosphine-catalyzed Suzuki-Miyaura coupling reactions
- Cu(II)-mediated Ullmann-type coupling
Reactant for preparation of:
- Alkynylphenoxyacetic acids as DP2 receptor antagonists for treatment of allergic inflammatory diseases
- Tetrahydrobenzothiophenes as conformationally restricted enol-mimic inhibitors of type II dehydroquinase via Paal-Knorr synthesis involving Suzuki coupling
- Highly substituted benzannulated cyclooctanol derivatives by samarium diiodide-mediated cyclization
- Stereospecific dienes via nickel-catalyzed three-component reductive coupling with alkynes and enones
Reactant for
Reactant for preparation of
- Palladium-phosphine-catalyzed Suzuki-Miyaura coupling reactions
- Cu(II)-mediated Ullmann-type coupling
- Palladium-catalyzed Sonogashira cross-coupling
Reactant for preparation of
- Alkynylphenoxyacetic acids as DP2 receptor antagonists for treatment of allergic inflammatory diseases
- Tetrahydrobenzothiophenes as conformationally restricted enol-mimic inhibitors of type II dehydroquinase via Paal-Knorr synthesis involving Suzuki coupling
- Highly substituted benzannulated cyclooctanol derivatives by samarium diiodide-mediated cyclization
- Stereospecific dienes via nickel-catalyzed three-component reductive coupling with alkynes and enones
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahydrobenzothiophene derivatives: conformationally restricted inhibitors of type II dehydroquinase.
Sonia Paz et al.
ChemMedChem, 6(2), 266-272 (2011-01-29)
The preparation of substituted pyrazoles from β,β-dibromo-enones by a tandem condensation/Suzuki-Miyaura cross-coupling process
Beltran-Rodil, S.; et al.
Synlett, 4, 602-606 (2010)
Highly substituted benzannulated cyclooctanol derivatives by samarium diiodide-induced cyclizations.
Jakub Saadi et al.
Beilstein journal of organic chemistry, 6, 1229-1245 (2011-02-02)
A series of γ-oxo esters suitably substituted with various styrene subunits was subjected to samarium diiodide-induced 8-endo-trig cyclizations. Efficacy, regioselectivity and stereoselectivity of these reactions via samarium ketyls strongly depend on the substitution pattern of the attacked alkene moiety. The
A new strategy for the synthesis of substituted dihydropyrones and tetrahydropyrones via palladium-catalyzed coupling of thioesters
Fuwa, H.; et al.
Tetrahedron, 67, 4995-5010 (2011)
Ming-Bo Zhou et al.
The Journal of organic chemistry, 75(16), 5635-5642 (2010-08-14)
Palladium-catalyzed cross-coupling reaction of terminal alkynes with arylboronic acids has been described. In the presence of Pd(OAc)(2) and Ag(2)O, a variety of terminal alkynes, including electron-poor terminal alkynes, smoothly underwent the reaction with numerous boronic acids to afford the corresponding
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service