49733
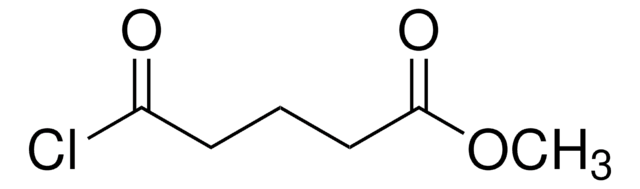
Glutaric acid monoethyl ester chloride
≥97.0% (NT)
Synonym(s):
Ethyl 4-(chloroformyl)butyrate, Ethyl 5-chloro-5-oxovalerate, Ethyl glutaryl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClCO(CH2)3COOC2H5
CAS Number:
Molecular Weight:
178.61
Beilstein:
1099817
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (NT)
refractive index
n20/D 1.443
bp
68-70 °C/0.07 mmHg (lit.)
functional group
acyl chloride
ester
storage temp.
2-8°C
SMILES string
CCOC(=O)CCCC(Cl)=O
InChI
1S/C7H11ClO3/c1-2-11-7(10)5-3-4-6(8)9/h2-5H2,1H3
InChI key
KKJAQUGGQMCNJY-UHFFFAOYSA-N
General description
Glutaric acid monoethyl ester chloride (ethyl glutaryl chloride) is a higher acyl chloride homolog. Its reaction with calix[4]arene to form ester group-containing calix[4]arenes has been reported.
Application
Glutaric acid monoethyl ester chloride may be used as a reagent during the multi-step synthesis of lipidic aminoalcohol and diamine derivatives.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Esther del Olmo et al.
Bioorganic & medicinal chemistry letters, 16(23), 6091-6095 (2006-09-27)
Lymphoproliferation inhibition and cytotoxicity of a number of lipidic aminoacids, aminoalcohols and diamines were evaluated as a preliminary screening to select potential immunomodulators. The four most potent/less toxic compounds were submitted to delayed hypersensibility (DTH) assays to define the best
Synthesis of Several Diester Group-Containing Calix [4] arenes.
Nam KC, et al.
Bull. Korean Chem. Soc., 17(6), 502-506 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service