495336
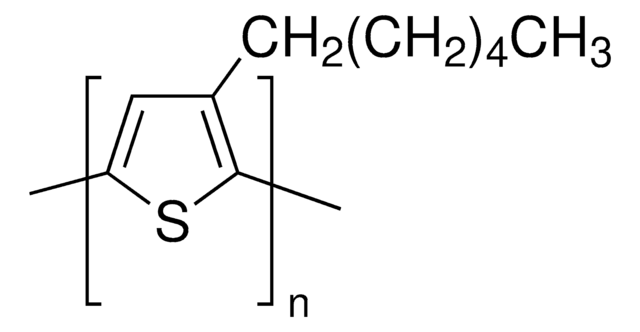
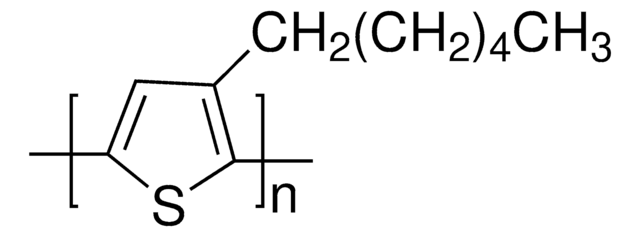
Poly(3-butylthiophene-2,5-diyl)
regioregular
Synonym(s):
P3BT
About This Item
Recommended Products
mol wt
Mw 54,000 (typical)
color
black
solubility
chlorinated solvents: soluble (partially soluble in THF, diethylether)
fluorescence
λex 440 nm; λem 567 nm in chloroform
Mw/Mn
2.3 (typical)
SMILES string
[s]1c(c(cc1C)CCCC)C
InChI
1S/C10H16S/c1-4-5-6-10-7-8(2)11-9(10)3/h7H,4-6H2,1-3H3
InChI key
DUOSBQJOYVIVOR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Packaging
Citation
Legal Information
Rieke is a registered trademark of Rieke Metals, Inc.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The application of conducting polymers at the interface with biology is an exciting new trend in organic electronics research.
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Novel Graphene‑Based Nanostructures Production, Functionalization, and Engineering
Recent progress in the area of solution-processed functional materials has led to the development of a variety of thin-film optoelectronic devices with significant promise in the industrial and consumer electronics fields.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Poly[(m-phenylenevinylene)-co-(2,5-dioctoxy-p-phenylenevinylene)] light-emitting polymer, predominantly trans](/deepweb/assets/sigmaaldrich/product/structures/249/040/9442b889-4fa0-4b4a-b424-cff0769a5ef2/640/9442b889-4fa0-4b4a-b424-cff0769a5ef2.png)