All Photos(1)
About This Item
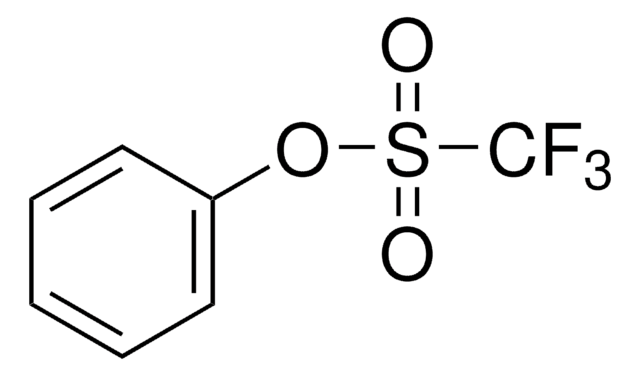
Empirical Formula (Hill Notation):
C7H9F3O3S
CAS Number:
Molecular Weight:
230.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
84-87 °C/4 mmHg (lit.)
density
1.315 g/mL at 25 °C (lit.)
functional group
fluoro
triflate
storage temp.
2-8°C
SMILES string
FC(F)(F)S(=O)(=O)OC1=CCCCC1
InChI
1S/C7H9F3O3S/c8-7(9,10)14(11,12)13-6-4-2-1-3-5-6/h4H,1-3,5H2
InChI key
WVSCRRLWRRANJY-UHFFFAOYSA-N
Related Categories
General description
1-Cyclohexenyl trifluoromethanesulfonate, also known as 1-cyclohexenyl triflate, is a cyclohexenyl sulfonate. Its trifluoromethylation reaction in the presence of different monodentate biaryl phosphine ligands has been investigated. The asymmetric Heck reaction of 1-cyclohexenyl trifluoromethanesulfonate using palladium complexes of phosphine oxazoline ligand has been studied.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
160.0 °F - closed cup
Flash Point(C)
71.1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Eun Jin Cho et al.
Organic letters, 13(24), 6552-6555 (2011-11-25)
A method for the palladium-catalyzed trifluoromethylation of cyclohexenyl sulfonates has been developed. Various cyclohexenyl triflates and nonaflates underwent trifluoromethylation under mild reaction conditions using a catalyst system composed of Pd(dba)(2) or [(allyl)PdCl](2) and the monodentate biaryl phosphine ligand (t)BuXPhos. The
Proline derived phosphine-oxazoline ligands in the asymmetric Heck reaction.
Gilbertson SR, et al.
Tetrahedron Letters, 42(3), 365-368 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)
![Thieno[3,2-b]thiophene 95%](/deepweb/assets/sigmaaldrich/product/structures/353/609/429fd4bf-e217-4371-80a3-9e5a4d88908b/640/429fd4bf-e217-4371-80a3-9e5a4d88908b.png)