374962
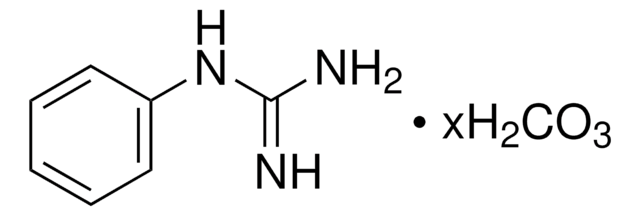
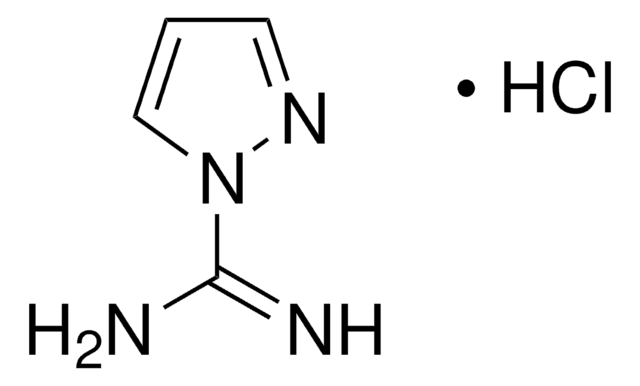
4-Guanidinobenzoic acid hydrochloride
99%
Synonym(s):
4-Guanidobenzoic acid hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
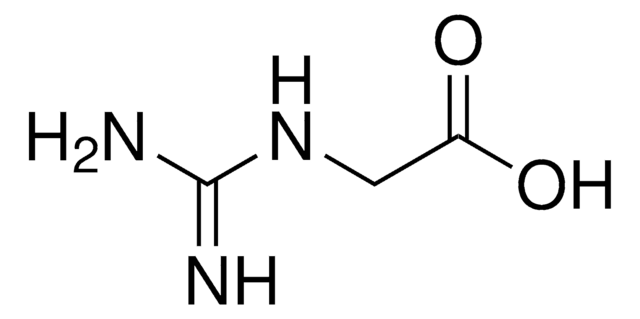
H2NC(=NH)NHC6H4CO2H · HCl
CAS Number:
Molecular Weight:
215.64
Beilstein:
3718032
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
285 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
Cl[H].NC(=N)Nc1ccc(cc1)C(O)=O
InChI
1S/C8H9N3O2.ClH/c9-8(10)11-6-3-1-5(2-4-6)7(12)13;/h1-4H,(H,12,13)(H4,9,10,11);1H
InChI key
YETFLAUJROGBMC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Guanidinobenzoic acid hydrochloride is a guanidine derivative. Quality standard of 4-guanidinobenzoic acid hydrochloride has been investigated.
Application
4-Guanidinobenzoic acid hydrochloride may be used in ELISA inhibition assay of mouse antiserum. It may be used in the synthesis of human acrosin inhibitor 4′-acetaminophenyl 4-guanidinobenzoate hydrochloride.2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Ookawara et al.
European journal of clinical pharmacology, 51(2), 149-151 (1996-01-01)
Nafamostat mesilate, a potent protease inhibitor, is widely used for the treatment of pancreatitis, disseminated intravascular coagulation and as an anticoagulant in haemodialysis. However, hyperkalaemia associated with nafamostat mesilate has been reported. It is thought to be due to decreased
M K Ramjee et al.
Thrombosis research, 98(6), 559-569 (2000-07-19)
Nafamostat mesilate (FUT-175), a synthetic serine protease inhibitor, is active against a number of the serine proteases involved in coagulation. This has been proposed as the basis of its anticoagulant activity. We investigated the reaction of Nafamostat with bovine pancreatic
I Midgley et al.
Xenobiotica; the fate of foreign compounds in biological systems, 24(1), 79-92 (1994-01-01)
1. The metabolic fate of N,N-dimethylcarbamoylmethyl 4-(4-guanidino[14C]benzoyloxy)phenylacetate methanesulphonate (14C-camostat mesylate) was investigated after i.v. administration to man (12-h infusion), and to rat and dog (bolus injection). 2. Renal excretion (mainly in 24 h) accounted for at least 80% dose in
Di-ya L, et al.
Chinese Pharmaceutical Journal, 3, 28-28 (2010)
S Muto et al.
British journal of pharmacology, 111(1), 173-178 (1994-01-01)
1. The present experiments were undertaken to determine the mechanism(s) of hyperkalaemia caused by nafamostat mesilate (NM), a serine-protease inhibitor. 2. We investigated the effects of luminal addition of two metabolites of NM, p-guanidinobenzoic acid (PGBA) and 6-amidino-2-naphthol (AN), on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service