374776
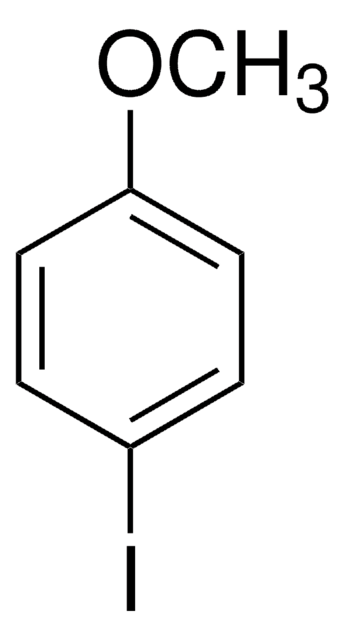
4-Methoxybiphenyl
97%
Synonym(s):
4-Phenylanisole
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H5C6H4OCH3
CAS Number:
Molecular Weight:
184.23
EC Number:
MDL number:
UNSPSC Code:
12171500
PubChem Substance ID:
NACRES:
NA.47
Recommended Products
Assay
97%
form
powder, crystals or chunks
mp
86-90 °C (lit.)
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
COc1ccc(cc1)-c2ccccc2
InChI
1S/C13H12O/c1-14-13-9-7-12(8-10-13)11-5-3-2-4-6-11/h2-10H,1H3
InChI key
RHDYQUZYHZWTCI-UHFFFAOYSA-N
Application
4-Methoxybiphenyl has been used as a standard reagent whose fluorescence intensity is associated with the fluorescence characteristics of the products of derivatization reaction for aryl halides with phenylboronic acid (PBA).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J R Fry
Xenobiotica; the fate of foreign compounds in biological systems, 17(6), 751-758 (1987-06-01)
1. The metabolism of 4-methoxybiphenyl to 4-hydroxybiphenyl and its sulphate and glucuronic acid conjugates has been studied in rat isolated hepatocytes at various concentrations of 4-methoxybiphenyl. 2. The proportions of metabolites produced remained constant at concentrations of 4-methoxybiphenyl less than
A comparison of biphenyl 4-hydroxylation and 4-methoxybiphenyl O-demethylation in rat liver microsomes.
J R Fry
Biochemical pharmacology, 30(14), 1915-1919 (1981-07-15)
P Paterson et al.
Xenobiotica; the fate of foreign compounds in biological systems, 15(6), 493-502 (1985-06-01)
The rate of production of 4-hydroxybiphenyl from 4-methoxybiphenyl in hepatocytes isolated from untreated rats was essentially identical to that from biphenyl in hepatocytes isolated from rats pretreated with beta-naphthoflavone at 40 mg/kg. Similar results were obtained using liver microsomes isolated
Influence of the sulphation inhibitor, 2,6-dichloro-4-nitrophenol, on the production and conjugation, of 4-hydroxybiphenyl generated from 4-methoxybiphenyl by rat isolated hepatocytes.
J R Fry et al.
Biochemical pharmacology, 36(18), 3090-3092 (1987-09-15)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service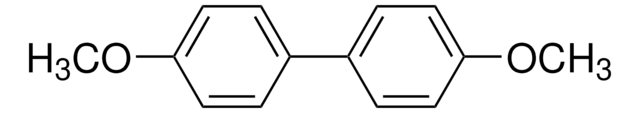


![1-(4′-Methyl[1,1′-biphenyl]-4-yl)ethanone AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/224/118/064dfb31-1067-44ce-bf18-d3f762028eb6/640/064dfb31-1067-44ce-bf18-d3f762028eb6.png)