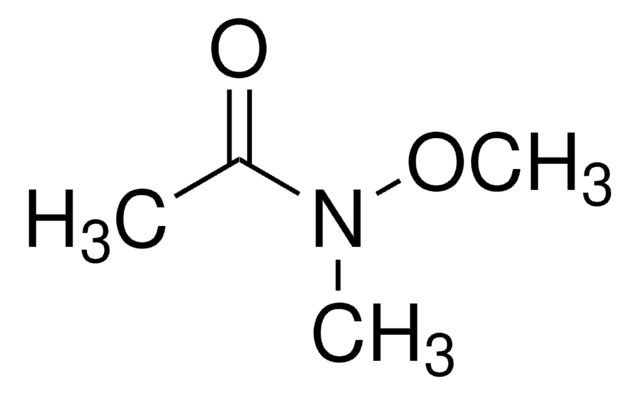
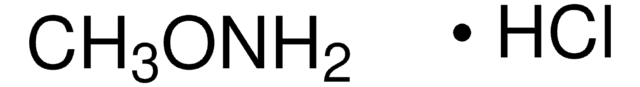All Photos(2)
About This Item
Linear Formula:
C6H5CON(OCH3)CH3
CAS Number:
Molecular Weight:
165.19
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.533 (lit.)
bp
70 °C/0.1 mmHg (lit.)
density
1.085 g/mL at 25 °C (lit.)
SMILES string
CON(C)C(=O)c1ccccc1
InChI
1S/C9H11NO2/c1-10(12-2)9(11)8-6-4-3-5-7-8/h3-7H,1-2H3
InChI key
UKERDACREYXSIV-UHFFFAOYSA-N
Related Categories
General description
N-Methoxy-N-methylbenzamide is an N,N-disubstituted benzamide. also referred as Weinreb amide., Hydrogen bonding interactions between thioacetamide and N-methoxy-N-methylbenzamide has been investigated using near-infrared absorption spectroscopy. Preparation of N-methoxy-N-methylbenzamide has been reported.
Application
N-Methoxy-N-methylbenzamide may be used in the preparation of β-trifluoromethyl enaminones.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
An efficient conversion of carboxylic acids into Weinreb amides.
Katritzky AR, et al.
ARKIVOC (Gainesville, FL, United States), 11, 39-44 (2002)
Near-infrared spectroscopic studies of the hydrogen bonding between thioacetamide and< i> N</i>,< i> N</i>-disubstituted benzamide derivatives in CCl< sub> 4</sub>.
Choi YS, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 52(13), 1779-1783 (1996)
A novel approach to ?-trifluoromethyl enaminones.
Jeong IH, et al.
Tetrahedron Letters, 43(40), 7171-7174 (2002)
Kazuhito Hioki et al.
Chemical & pharmaceutical bulletin, 52(4), 470-472 (2004-04-02)
Weinreb amides were successfully prepared from the corresponding carboxylic acids using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM) in the solvents, methanol, isopropyl alcohol, and acetonitrile, which can solubilize DMT-MM. A variety of carboxylic acids were converted to the corresponding Weinreb amides in excellent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service