All Photos(3)
About This Item
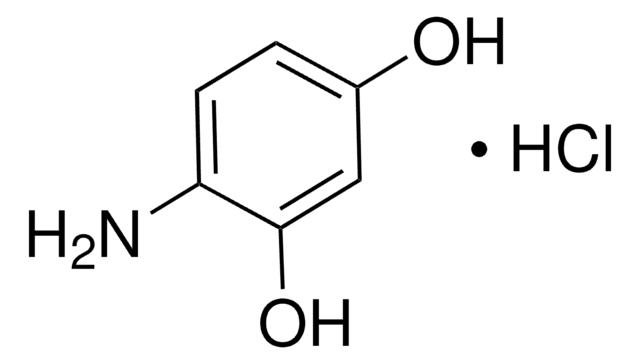
Linear Formula:
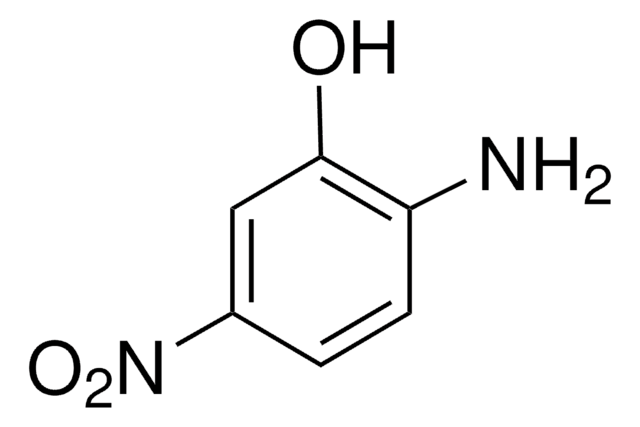
(H2N)2C6H3OH
CAS Number:
Molecular Weight:
124.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
161-165 °C (lit.)
SMILES string
Nc1cccc(O)c1N
InChI
1S/C6H8N2O/c7-4-2-1-3-5(9)6(4)8/h1-3,9H,7-8H2
InChI key
PCAXITAPTVOLGL-UHFFFAOYSA-N
General description
2,3-Diaminophenol is an aromatic diamine and forms Pd(II) and Pt(II) complexes. 2,3-Diaminophenol reacts with 2,4-pentanedione to yield the corresponding benzo[b][1,4]diazepinium salts. 2,3-Diaminophenol reacts with salicylaldehyde or 5-bromosalicylaldehyde in absolute ethanol to yield new unsymmetrical Schiff base.
Application
2,3-Diaminophenol was used:
- in one-pot microwave assisted synthesis of amino-1,5-benzoxazepines and hydroxyl-1,5-benzodiazepines
- in the electrosynthesis of poly(2,3-diaminophenol) via electro-oxidation
- in a synthesis of tetradentate Schiff base complexes via reaction with salicylaldehyde or 5-bromosalicylaldehyde and metals such as Mn(III), Ni(II) and Cu(II)
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Unsymmetrical tetradentate Schiff base complexes derived from 2, 3-diaminophenol and salicylaldehyde or 5-bromosalicylaldehyde.
Ourari A, et al.
Transition Metal Chemistry, 31(2), 169-175 (2006)
Transition Met. Chem. (London), 31, 169-169 (2006)
Electropolymerization of 2, 3-diaminophenol.
Del Valle MA, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 38(9), 1698-1703 (2000)
Andreas Schmidt et al.
Organic & biomolecular chemistry, 1(23), 4342-4350 (2003-12-20)
2,3-Diaminophenol 4, 3,4-diaminophenol 5, 4-methoxy-1,2-diaminobenzene 6, 3,4-diaminobenzenethiol 7, 2,3-diaminobenzoic acid 8, and 3,4-diaminobenzoic acid 9 were reacted with 2,4-pentanedione to yield the corresponding benzo[b][1,4]diazepinium salts, respectively. The hydroxy-benzo[b][1,4]diazepinium salts 17 and 18 do not form mesomeric betaines (MB) on deprotonation.
Constantinos G Neochoritis et al.
Journal of medicinal chemistry, 53(23), 8409-8420 (2010-11-06)
Amino-1,5-benzoxazepines 2 and 5 and hydroxyl-1,5-benzodiazepines 3 and 6 have been synthesized in one-pot solvent-free conditions from 2,3-diaminophenol and ketones through microwave assisted acid catalysis, the benzoxazepine/benzodiazepine ratio depending on the R(1) and R(3) aryl substituents. The otherwise inaccessible and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service