328464
2-Azetidinone
98%
Synonym(s):
β-Propiolactam, 2-Azacyclobutanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H5NO
CAS Number:
Molecular Weight:
71.08
Beilstein:
104563
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
106 °C/15 mmHg (lit.)
mp
74-76 °C (lit.)
solubility
chloroform: very soluble(lit.)
ethanol: very slightly soluble(lit.)
water: very soluble(lit.)
storage temp.
2-8°C
SMILES string
O=C1CCN1
InChI
1S/C3H5NO/c5-3-1-2-4-3/h1-2H2,(H,4,5)
InChI key
MNFORVFSTILPAW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Azetidinone is an unsubstituted four membered lactam. 2-Azetidinone is the fundamental building block of β-lactam antibiotics. X-ray photoelectron spectra of 2-azetidinone was investigated. 2-Azetidinone undergoes hydrolysis with aqueous alkali to yield β-alanine.
Application
2-Azetidinone was used in synthesis of optically pure densely functionalized γ-lactams.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Benito Alcaide et al.
The Journal of organic chemistry, 69(3), 993-996 (2004-01-31)
A synthesis of optically pure densely functionalized gamma-lactams starting from 2-azetidinone-tethered iminophosphoranes has been developed. Full chirality transfer has been accomplished from the enantiomerically pure 2-azetidinones. The addition of lithium acetylides to 4-oxoazetidine-2-carbaldehydes at -78 degrees C smoothly yielded propargylic
Investigation of the molecular structure and VUV-induced ion dissociation dynamics of 2-azetidinone (C3 H5 NO).
Alexsandre F Lago et al.
Rapid communications in mass spectrometry : RCM, 35(3), e8988-e8988 (2020-10-24)
2-Azetidinone (?-propiolactam)
Holley RW and Holley AD.
Journal of the American Chemical Society, 71(6), 2129-2131 (1949)
Marawan Ahmed et al.
The journal of physical chemistry. A, 116(33), 8653-8660 (2012-07-18)
X-ray photoelectron spectra of the core and valence levels of the fundamental building blocks of β-lactam antibiotics have been investigated and compared with theoretical calculations. The spectra of the compounds 2-azetidinone and the 2- and 4-isomers of thiazolidine-carboxylic acid are
Tim N Beck et al.
Bioorganic & medicinal chemistry, 23(3), 632-647 (2015-01-01)
The prevalence of drug resistance in both clinical and community settings as a consequence of alterations of biosynthetic pathways, enzymes or cell wall architecture is a persistent threat to human health. We have designed, synthesized, and tested a novel class
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service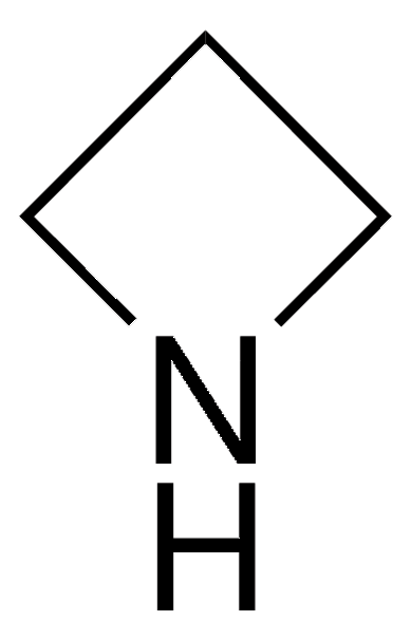



![2-Oxa-6-azaspiro[3.3]heptane](/deepweb/assets/sigmaaldrich/product/structures/391/874/ff74bb51-dd44-4cca-9b3f-3b380ccae360/640/ff74bb51-dd44-4cca-9b3f-3b380ccae360.png)