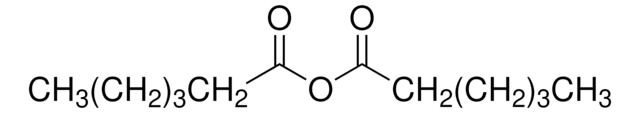
245933
Valeric anhydride
97%
Synonym(s):
Pentanoic anhydride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
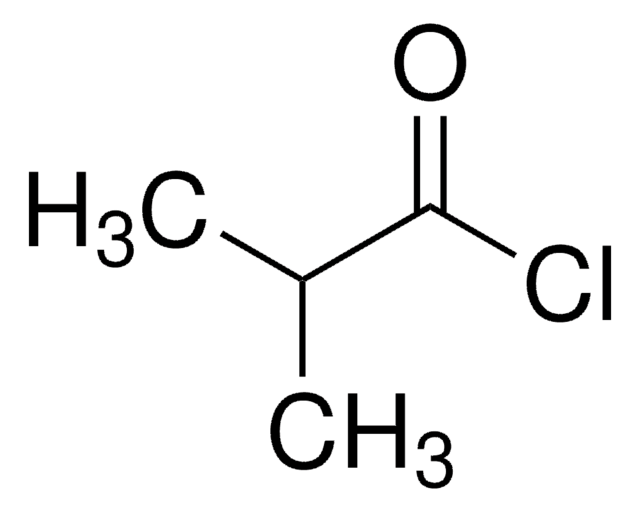
Linear Formula:
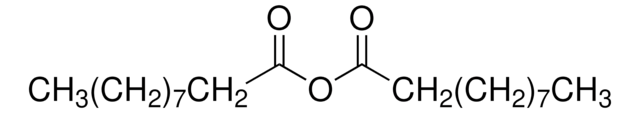
[CH3(CH2)3CO]2O
CAS Number:
Molecular Weight:
186.25
Beilstein:
1770130
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.421 (lit.)
bp
228-230 °C (lit.)
mp
−56 °C (lit.)
density
0.944 g/mL at 20 °C (lit.)
SMILES string
CCCCC(=O)OC(=O)CCCC
InChI
1S/C10H18O3/c1-3-5-7-9(11)13-10(12)8-6-4-2/h3-8H2,1-2H3
InChI key
DUCKXCGALKOSJF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Valeric anhydride can be used as a reactant to synthesize:
- Alkyl 9-nitrocamptothecin esters by the esterification reaction.
- Modified bismuth metal-organic frameworks (Bi-MOFs).
- O-acylated chitosan nanofibers (CSNFs) for potential usage in biomaterials and food packaging.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
210.2 °F - closed cup
Flash Point(C)
99 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
O-acylation of chitosan nanofibers by short-chain and long-chain fatty acids
Zhang Z, et al.
Carbohydrate Polymers, 177, 203-209 (2017)
Zhi-Song Cao et al.
Acta pharmacologica Sinica, 24(2), 109-119 (2003-01-28)
To study the structure-activity relationship of alkyl 9-nitrocamptothecin esters. Two alkyl 9-nitrocamptothecin (9NC) esters 5g and 5h were prepared by esterification reactions of 9NC with valeric anhydride and heptanoic anhydride, respectively. Eight 9NC esters 5a-5h were tested for cytotoxicity against
Synthesis, functionalisation and post-synthetic modification of bismuth metal-organic frameworks
Koppen M, et al.
Dalton Transactions, 46(26), 8658-8663 (2017)
Karolina Skołucka-Szary et al.
Materials science & engineering. C, Materials for biological applications, 55, 50-60 (2015-06-29)
In this article, the synthesis of novel biopolymer, chitin dipentanoate (Di-O-Valeryl Chitin, DVCH) has been described. DVCH is a chitin derivative esterified with two valeryl groups at positions 3 and 6 of the N-acetylglucosamine units and it is soluble in
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 245933-100ML | 4061825849782 |
| 245933-500ML | 4061836691615 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service