All Photos(1)
About This Item
Empirical Formula (Hill Notation):
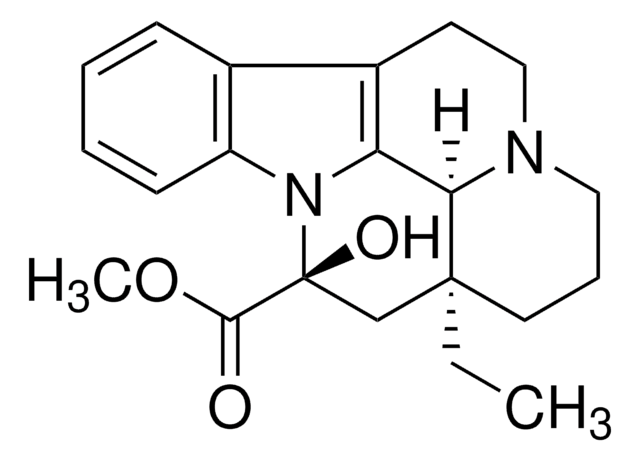
C21H26N2O3
CAS Number:
Molecular Weight:
354.44
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
optical activity
[α]23/D +42.8°, c = 1 in pyridine
mp
232 °C (dec.) (lit.)
SMILES string
CC[C@@]12CCCN3CCc4c(C13)n(c5ccccc45)[C@](O)(C2)C(=O)OC
InChI
1S/C21H26N2O3/c1-3-20-10-6-11-22-12-9-15-14-7-4-5-8-16(14)23(17(15)18(20)22)21(25,13-20)19(24)26-2/h4-5,7-8,18,25H,3,6,9-13H2,1-2H3/t18-,20+,21+/m1/s1
InChI key
RXPRRQLKFXBCSJ-GIVPXCGWSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Vincamine can be used as a starting material to synthesize:
- Vincamine acid, which is employed as a precursor in the synthesis of vinpocetine by dehydration and esterification using sulfuric acid.
- Apovincamine using iron(III) perchlorate.
- (-)-Criocerine via one-step iodination reaction.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L Vereczkey
European journal of drug metabolism and pharmacokinetics, 10(2), 89-103 (1985-04-01)
The pharmacokinetics and metabolism of vincamine, vinpocetine, methylene-methoxy-apovincaminic acid ester and eburnamine have been reviewed. The main route of elimination for vincamine, vinpocetine and methylene-methoxy-apovincaminic acid ester is ester cleavage and conjugation in the case of eburnamine. Vincamine and its
L Bezin et al.
Brain research. Molecular brain research, 76(2), 275-281 (2000-04-14)
The number of tyrosine hydroxylase (TH)-expressing neurons appears to be precisely determined in basal conditions within the noradrenergic pontine nucleus locus coeruleus (LC). However, additional neurons exhibiting TH phenotype have been observed in the adult rat LC following a single
Ying-Ping Juan et al.
Journal of chromatography. A, 1088(1-2), 146-151 (2005-09-01)
Vincamine is an alkaloid compound derived from the Vinca minor plant. Since little is known concerning its pharmacokinetics and appropriate analytical method, this study focuses on its pharmacokinetics as well the possible roles of the multidrug transporter P-glycoprotein on its
Istvan Moldvai et al.
The Journal of organic chemistry, 71(10), 3768-3772 (2006-05-06)
beta-Iodo-enamines with an eburnane skeleton (5a and 5c) were obtained with the aid of iodine from compounds 2a and 2c and were then transformed into hydroxyl lactams (6a and 6c) with CuSO4.5H2O in a mixture of DMF and water. Lactams
Li Di et al.
Journal of biomolecular screening, 11(1), 40-47 (2005-10-20)
Screening of solution stability provides an early alert on potential liabilities of drug candidates so that strategies can be developed to overcome the challenges. A fully automated solution stability assay has been developed to accelerate traditional manual operation. The assay
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service