All Photos(2)
About This Item
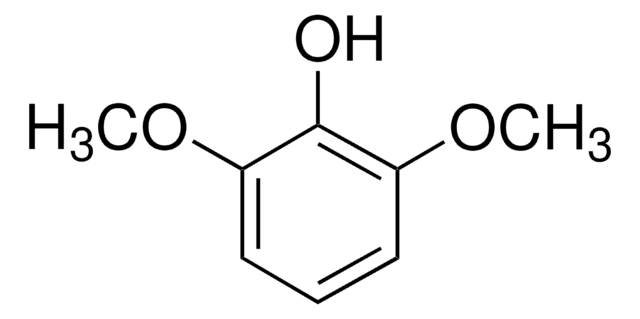
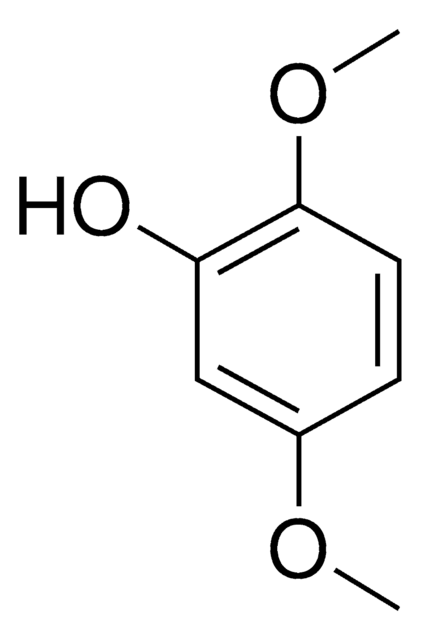
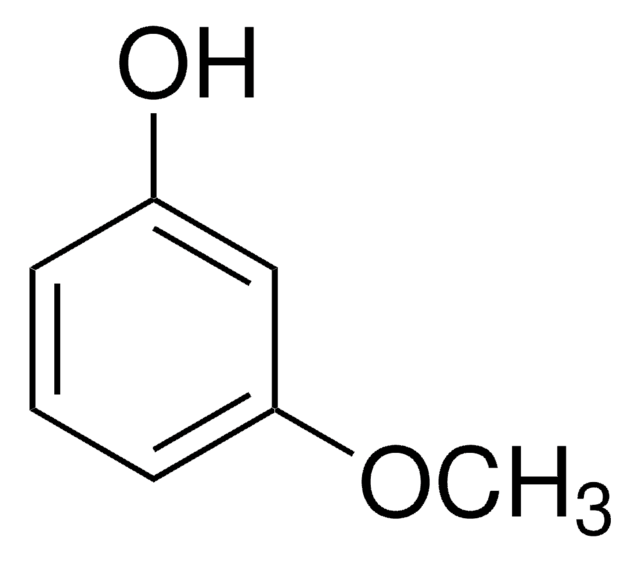
Linear Formula:
(CH3O)2C6H3OH
CAS Number:
Molecular Weight:
154.16
Beilstein:
1366650
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
79-82 °C (lit.)
SMILES string
COc1ccc(O)cc1OC
InChI
1S/C8H10O3/c1-10-7-4-3-6(9)5-8(7)11-2/h3-5,9H,1-2H3
InChI key
SMFFZOQLHYIRDA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,4-Dimethoxyphenol was used in the synthesis of:
- 5,6-dimethoxy benzofuranone derivatives, multi-target anti Alzheimer compounds
- 3,4-dimethoxyphenyl-β-D-glucopyranoside
- 4-(but-2-enyloxy)-1,2-dimethoxybenzene
- precursors for the synthesis of the 4H-chromenes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hamid Nadri et al.
Daru : journal of Faculty of Pharmacy, Tehran University of Medical Sciences, 21(1), 15-15 (2013-03-01)
Several studies have been focused on design and synthesis of multi-target anti Alzheimer compounds. Utilizing of the dual Acetylcholinesterase/Butyrylcholinesterase inhibitors has gained more interest to treat the Alzheimer's disease. As a part of a research program to find a novel
Ring-closing metathesis for the synthesis of 2H-and 4H-chromenes.
van Otterlo WAL, et al.
Tetrahedron, 61(42), 9996-10006 (2005)
H Takii et al.
Bioscience, biotechnology, and biochemistry, 61(9), 1531-1535 (1997-10-27)
Glycosides were screened for their lowering effect on the postprandial blood glucose rise in vivo. The effect of phlorizin and other phenolic glycosides on the postprandial blood glucose response to glucose ingestion was evaluated in Std ddY mice. When phlorizin
Peter Lorenz et al.
Chemistry & biodiversity, 15(5), e1800035-e1800035 (2018-03-27)
Seeds from Hypericum species have recently been identified as an interesting source of xanthone derivatives. Extraction of seeds from H. perforatum with MeOH and subsequent concentration via polyamide adsorption yielded a fraction enriched in tetrahydroxyxanthones (THX), which were further semipurified
M N M Cunha et al.
Colloids and surfaces. B, Biointerfaces, 154, 210-220 (2017-03-28)
Silver nanoparticles (AgNPs) were synthesized by citrate reduction method in the presence of polymers, poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA) and chitosan, used as stabilizing agents, and an oxidoreductase enzyme, laccase (Lac), with the goal of expanding the NPs antimicrobial
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service