193739
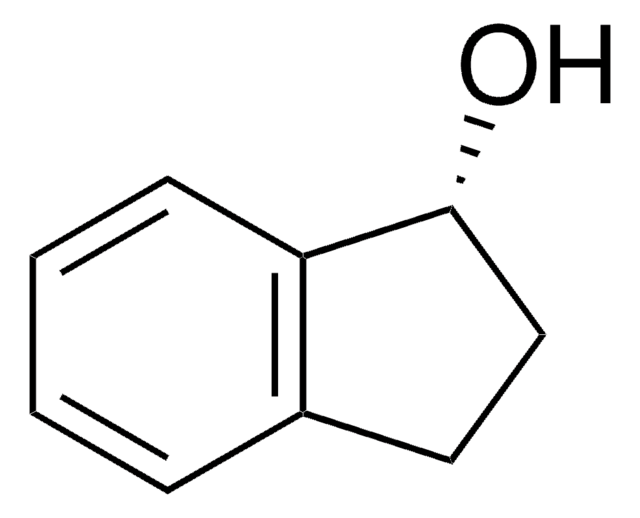
1-Indanol
98%
Synonym(s):
(±)-1-Indanol, (±)-1-Hydroxyindan
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H10O
CAS Number:
Molecular Weight:
134.18
Beilstein:
2042960
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
128 °C/12 mmHg (lit.)
mp
50-54 °C (lit.)
SMILES string
OC1CCc2ccccc12
InChI
1S/C9H10O/c10-9-6-5-7-3-1-2-4-8(7)9/h1-4,9-10H,5-6H2
InChI key
YIAPLDFPUUJILH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Chiral-sensitive aggregation of 1-indanol has been studied by FTIR spectroscopy. Transfer dehydrogenation of 1-indanol has been investigated over heterogeneous palladium catalyst using cyclohexene as hydrogen acceptor.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wanda Mączka et al.
Molecules (Basel, Switzerland), 24(23) (2019-12-01)
The main purpose of this publication was to obtain the S-enantiomer of indan-1-ol with high enantiomeric excess and satisfactory yield. In our research, we used carrot callus cultures (Daucuscarota L.), whereby the enzymatic system reduced indan-1-one and oxidized indan-1-ol. During
Jonas Altnöder et al.
Physical chemistry chemical physics : PCCP, 15(25), 10167-10180 (2013-05-15)
The aggregation behavior of racemic and enantiopure 1-indanol has been studied by FTIR spectroscopy, resonant ion dip IR spectroscopy, and spontaneous Raman scattering in supersonic jets. This triple experimental approach, augmented by homology to related molecular fragments and dispersion-corrected DFT
Selective transfer dehydrogenation of aromatic alcohols on supported palladium.
Keresszegi C, et al.
New. J. Chem., 25(9), 1163-1167 (2001)
Alexandre de Saint Germain et al.
Phytochemistry, 168, 112112-112112 (2019-09-10)
Strigolactone (SL) plant hormones control plant architecture and are key players in both symbiotic and parasitic interactions. GR24, a synthetic SL analog, is the worldwide reference compound used in all bioassays for investigating the role of SLs in plant development
Conformational landscapes and free-jet rotational spectrum of indan-1-ol.
Biagio Velino et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 7(3), 565-568 (2006-02-14)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service