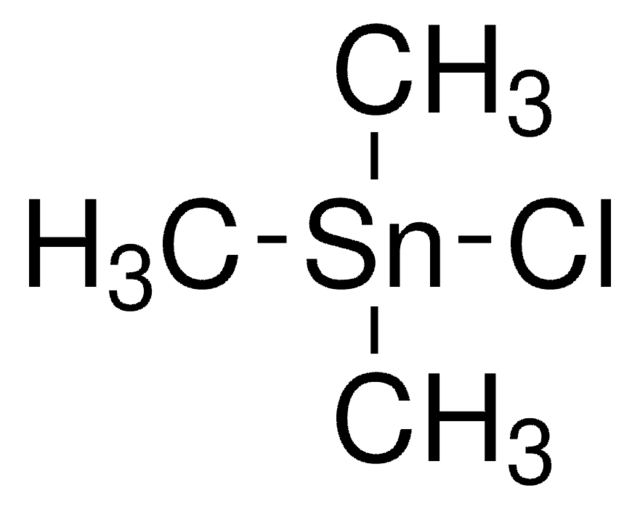186155
Lithium acetylide, ethylenediamine complex
90%
Synonym(s):
Lithium acetylide ethylenediamine complex, Lithium acetylide, compd. with 1,2-ethanediamine
About This Item
Recommended Products
Quality Level
Assay
90%
bp
110.6 °C (lit.)
mp
76 °C (dec.)
storage temp.
2-8°C
SMILES string
[Li]C#C.NCCN
InChI
1S/C2H8N2.C2H.Li/c3-1-2-4;1-2;/h1-4H2;1H;
InChI key
QJQWXTYPTBEPGS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Lithium acetylide, ethylenediamine complex is a general reagent to synthesize ethynylated ketones.
- It is used as a ligand in the coordination chemistry to synthesize transition metal complexes such as 1-alkynyl-dimethyl(triorganophosphine)gold(III) complex.
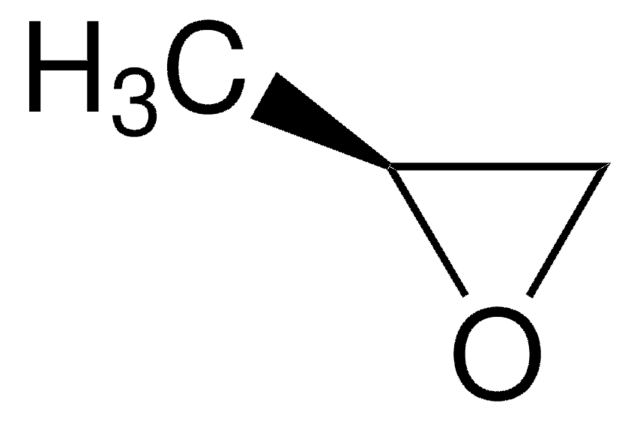
- It is employed in the ring opening reaction of epoxides, as in the total synthesis of (−)-goniotrionin and englerin A.
- It can also be used in the ethynylation of alkyl halides to prepare terminal alkynes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - Water-react 2
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service