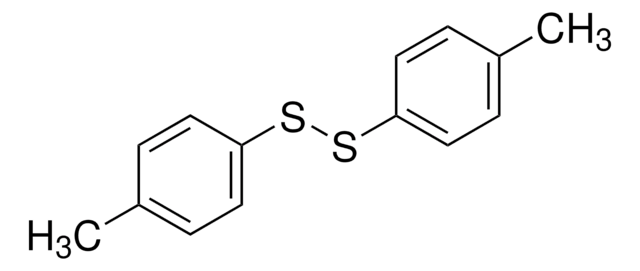
169021
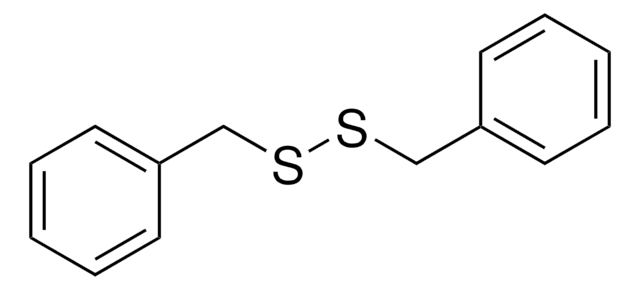
Phenyl disulfide
99%
Synonym(s):
Diphenyl disulfide, NSC 2689
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
C6H5SSC6H5
CAS Number:
Molecular Weight:
218.34
Beilstein:
639794
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
58-60 °C (lit.)
solubility
xylene: soluble 3%, clear, colorless to yellow
SMILES string
S(Sc1ccccc1)c2ccccc2
InChI
1S/C12H10S2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10H
InChI key
GUUVPOWQJOLRAS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Phenyl disulfide is used as a precursor for the synthesis of phenyl selenosulfide (PhS-SePh), which is vital in Li-ion battery production.
Application
Phenyl disulfide is the hydrolysis product of dyfonate( insecticide). Phenyl disulfide (diphenyl disulphide) participates in hydrothiolation of alkynes via amine-mediated single electron transfer mechanism.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Identification of hydrolytic metabolites of dyfonate in alkaline aqueous solutions by using high performance liquid chromatography-UV detection and gas chromatography-mass spectrometry.
Wang T, et al.
International Journal of Environmental Analytical Chemistry, 90(12), 948-961 (2010)
Tsuyoshi Taniguchi et al.
Organic letters, 11(15), 3298-3301 (2009-09-02)
Hydrothiolation of alkynes proceeds with diphenyl disulfide and tripropylamine. Amine-mediated single electron transfer to diphenyl disulfide can be proposed for the reaction mechanism. Applications of the method to radical cyclizations of eneyne compounds are also presented.
J M Young et al.
Agents and actions, 21(3-4), 314-315 (1987-08-01)
Indomethacin was administered subcutaneously to rats, 4 mg/kg/day for 4 consecutive days in order to produce erosions of the small intestine which were scored at necropsy on day 5. Orally administered phenidone (up to 250 mg/kg/day), a mixed cycloocygenase-lipoxygenase inhibitor
Y Ito et al.
Mutation research, 393(3), 307-316 (1997-12-11)
The suppressive effect of S-methyl methanethiosulfonate (MMTS) on aflatoxin B1 (AFB1)- or methyl methanesulfonate (MMS)-induced chromosome aberrations (CA) in rat bone marrow cells was studied. MMTS significantly suppressed CA induced by both AFB1 (an indirect-acting carcinogen) and MMS (a direct-acting
Y K Nakamura et al.
Mutation research, 385(1), 41-46 (1997-12-31)
S-Methyl methanethiosulfonate (MMTS) and diphenyl disulfide (DPDS) are temporary enzyme-sulfhydryl blocking agents. They are naturally occurring phytoalexin-like and synthetic substances known to be very potent bio-antimutagens in Escherichia coli B/r WP2. In the present paper, the suppressing effects of MMTS
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service