All Photos(1)
About This Item
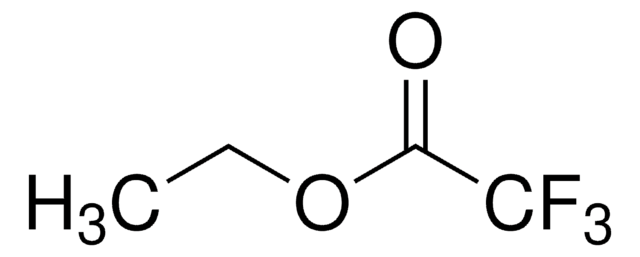
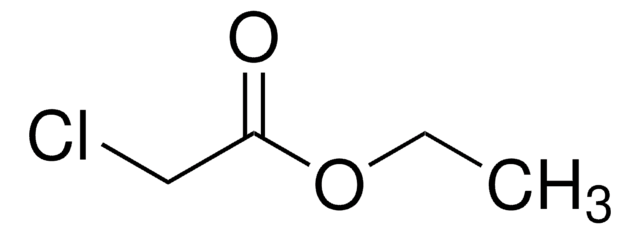
Linear Formula:
Cl3CCO2CH2CH3
CAS Number:
Molecular Weight:
191.44
Beilstein:
1761567
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.453 (lit.)
bp
168 °C (lit.)
solubility
alcohol: miscible(lit.)
diethyl ether: miscible(lit.)
water: insoluble(lit.)
density
1.378 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C(Cl)(Cl)Cl
InChI
1S/C4H5Cl3O2/c1-2-9-3(8)4(5,6)7/h2H2,1H3
InChI key
SJMLNDPIJZBEKY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ethyl trichloroacetate undergoes atom-transfer radical addition reaction with styrene catalyzed by the Ru-pentamethylcyclopentadienyl complexes.
Application
Ethyl trichloroacetate was used in the synthesis of dichlorocarbon precursor, phenyl(trichloromethyl)methyl.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
163.4 °F - closed cup
Flash Point(C)
73 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mariano A Fernández-Zúmel et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(43), 11601-11607 (2009-09-15)
Kinetic and spectroscopic analyses were performed to gain information about the mechanism of atom-transfer radical reactions catalyzed by the complexes [RuCl2Cp*(PPh3)] and [RuClCp*(PPh3)2] (Cp*=pentamethylcyclopentadienyl), in the presence and in the absence of the reducing agent magnesium. The reactions of styrene
An Improved Synthesis of Phenyl (trichloro-methyl) mercury from Sodium Methoxide and Ethyl Trichloroacetate.
Schweizer EE and O'Neill GJ.
The Journal of Organic Chemistry, 28(3), 851-852 (1963)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service