All Photos(1)
About This Item
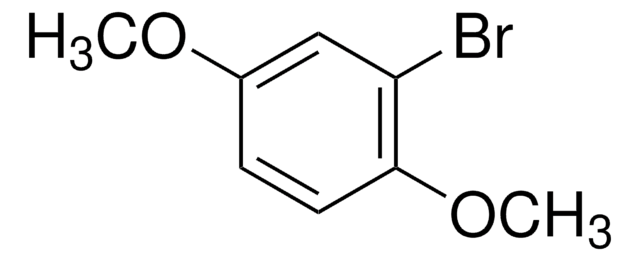
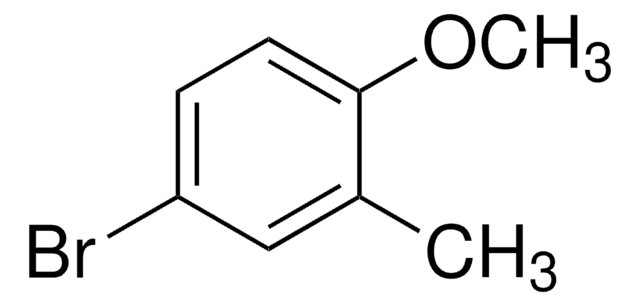
Linear Formula:
BrC6H3(OCH3)2
CAS Number:
Molecular Weight:
217.06
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.572 (lit.)
bp
153-155 °C/18 mmHg (lit.)
mp
25-26 °C (lit.)
density
1.507 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
COc1ccc(Br)c(OC)c1
InChI
1S/C8H9BrO2/c1-10-6-3-4-7(9)8(5-6)11-2/h3-5H,1-2H3
InChI key
NIUZVSQOXJIHBL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1-Bromo-2,4-dimethoxybenzene was used in the synthesis of 2,3-disubstituted benzo[b]furans. It was also used in the synthesis of dendrimer Si[CH2CH2Si(Me)2-2,4-(MeO)2-C6H3]4.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Selective lithiation and crystal structures of G1-carbosilane dendrimers with dimethoxybenzene functionalities.
Harder S, et al.
Journal of Organometallic Chemistry, 689(7), 1095-1101 (2004)
Dawei Yue et al.
The Journal of organic chemistry, 70(25), 10292-10296 (2005-12-06)
[reaction: see text] 2,3-Disubstituted benzo[b]furans are readily prepared under very mild reaction conditions by the palladium/copper-catalyzed cross-coupling of various o-iodoanisoles and terminal alkynes, followed by electrophilic cyclization with I2, PhSeCl, or p-O2NC6H4SCl. Aryl- and vinylic-substituted alkynes undergo electrophilic cyclization in
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 157554-25G | 4061838743404 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service