All Photos(1)
About This Item
Empirical Formula (Hill Notation):
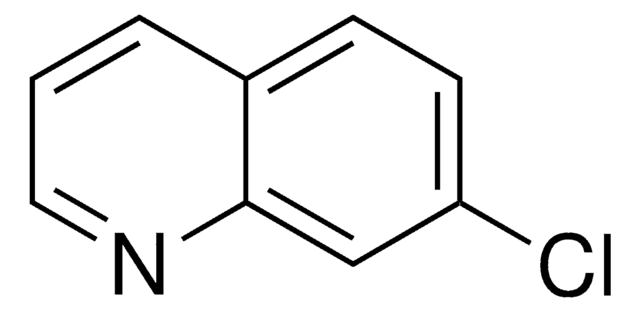
C10H8ClN
CAS Number:
Molecular Weight:
177.63
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.622 (lit.)
bp
269-270 °C (lit.)
density
0.881 g/mL at 25 °C (lit.)
functional group
chloro
SMILES string
Cc1cc(Cl)c2ccccc2n1
InChI
1S/C10H8ClN/c1-7-6-9(11)8-4-2-3-5-10(8)12-7/h2-6H,1H3
InChI key
HQAIROMRVBVWSK-UHFFFAOYSA-N
Application
4-Chloroquinaldine was used in the preparation of 4-phenyl-hydrazinoquinaldine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
208. The reaction between phenylhydrazine and 4-chloroquinoline derivatives, and the preparation of the corresponding 4-benzeneazo-and 4-amino-compounds.
Backeberg OG.
Journal of the Chemical Society, 1083-1087 (1938)
K N Vennila et al.
Bioorganic chemistry, 81, 184-190 (2018-08-24)
The induced fit docking of anilino quinoline scaffold results in the required hydrogen bonding interactions with amino acid residues in the orthosteric site of 3 Phosphoinositide dependent kinase (PDK1). The rational design of 4-substituted amino quinolines is carried out and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service