All Photos(2)
About This Item
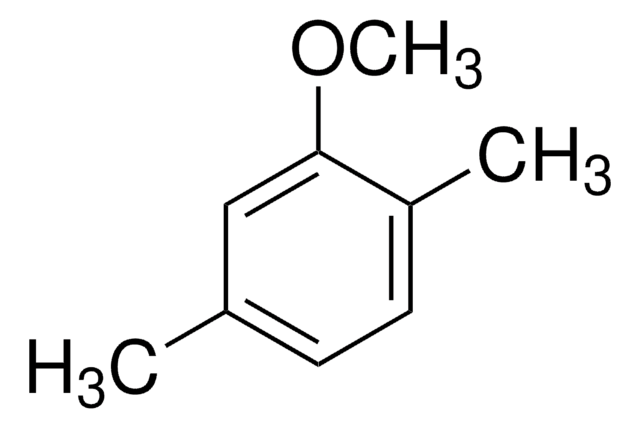
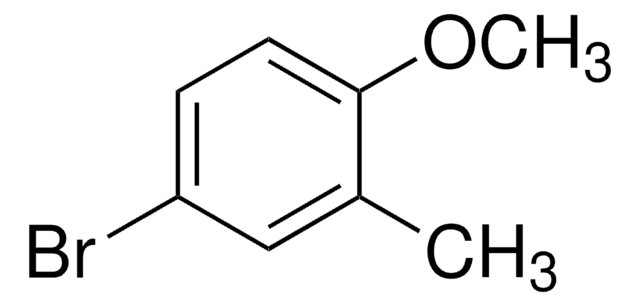
Linear Formula:
CH3C6H4OCH3
CAS Number:
Molecular Weight:
122.16
Beilstein:
1237336
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
5.25 mmHg ( 50 °C)
Assay
99%
form
liquid
refractive index
n20/D 1.511 (lit.)
bp
174 °C (lit.)
density
0.969 g/mL at 25 °C (lit.)
SMILES string
COc1ccc(C)cc1
InChI
1S/C8H10O/c1-7-3-5-8(9-2)6-4-7/h3-6H,1-2H3
InChI key
CHLICZRVGGXEOD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Methylanisole was used to prepare 5-methoxy-1,8-dimethyltetralin. It was also used as the solubilizate molecule to study the site of incorporation of solubilizates in sodium dodecyl sulfate (SDS) micellar systems.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3 - Repr. 2 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
138.2 °F - closed cup
Flash Point(C)
59 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Suratkar et al.
Journal of colloid and interface science, 225(1), 32-38 (2000-04-18)
The site of incorporation of solubilizates in sodium dodecyl sulfate (SDS) micellar systems has been investigated by proton NMR spectroscopy. The solubilizate molecules chosen for the present study are phenol, 4-methylphenol, 4-allyl-2-methoxyphenol, anisole, 4-methylanisole, 4-propenylanisole, 1,8-cineole, and limonene. These molecules
Brant L Kedrowski et al.
The Journal of organic chemistry, 73(13), 5177-5179 (2008-05-30)
A simple synthesis of the natural product cacalol has been developed that proceeds in seven steps and 21-25% overall yield. Ortho-lithiation of 4-methylanisole and alkylation with 5-iodo-1-pentene, followed by intramolecular Friedel-Crafts alkylation, gave 5-methoxy-1,8-dimethyltetralin. This compound was then formylated in
B Brunsborg et al.
Toxicology letters, 73(3), 209-212 (1994-09-01)
4-Methoxytoluene was given by gavage to 4 groups of 20 rats at dosage levels of 0, 40, 120 or 240 mg kg-1 body weight/day for 4 weeks. There was a statistically significant decrease in serum creatinine and urea in the
Occurrence of aromatic methyl migration (NIH-shift) during oxidation of p-methylanisole by hemin-thiolester complex as a cytochrome P-450 model.
H Sakurai et al.
Biochemical and biophysical research communications, 108(4), 1649-1654 (1982-10-29)
Jiangou Huang et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 67(3-4), 824-829 (2006-11-01)
One-color (1C), two-color (2C) resonant two-photon ionization (R2PI), and mass analyzed threshold ionization (MATI) methods have been applied to study the S(1)<--S(0) transition and threshold ionization of p-methylanisole. The excitation energy of the S(1)<--S(0) transition is determined to be 35,401+/-2
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service