All Photos(1)
About This Item
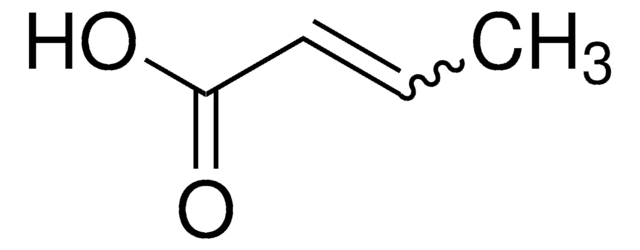
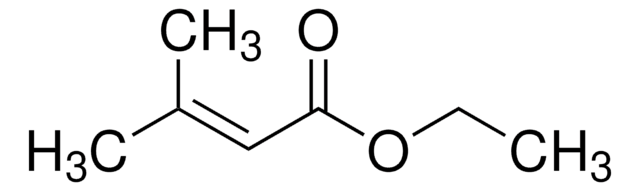
Linear Formula:
CH3CH=CHCOOCH3
CAS Number:
Molecular Weight:
100.12
Beilstein:
1720292
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.423 (lit.)
bp
118-120 °C (lit.)
density
0.944 g/mL at 25 °C (lit.)
SMILES string
COC(=O)\C=C\C
InChI
1S/C5H8O2/c1-3-4-5(6)7-2/h3-4H,1-2H3/b4-3+
InChI key
MCVVUJPXSBQTRZ-ONEGZZNKSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Methyl crotonate undergoes vinylogous aldol reaction with enolizable aldehydes in the presence of aluminum tris(2,6-di-2-naphthylphenoxide).
Application
Methyl crotonate was used to investigate chemoselectivity in the reaction between methyl crotonate and benzylamine catalyzed by lipase B from Candida antarctica using solvent engineering. It was used as starting reagent during the total synthesis of phytotoxins solanapyrones D(1) and E(2).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
30.2 °F - closed cup
Flash Point(C)
-1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jeffrey A Gazaille et al.
Organic letters, 14(11), 2678-2681 (2012-05-25)
The synthesis of the novel Lewis acid, aluminum tris(2,6-di-2-naphthylphenoxide) (ATNP), and its use in the vinylogous aldol reaction between methyl crotonate and enolizable aldehydes are described. ATNP is related to Yamamoto's Lewis acid, aluminum tris(2,6-diphenylphenoxide) (ATPH), but the 2-naphthyl groups
Solvent engineering: an effective tool to direct chemoselectivity in a lipase-catalyzed Michael addition.
Priego J, et al.
Tetrahedron, 65(2), 536-539 (2009)
Hisahiro Hagiwara et al.
The Journal of organic chemistry, 67(17), 5969-5976 (2002-08-17)
The phytotoxins solanapyrones D (1) and E (2) have been synthesized from the decalone prepared by the domino Michael reaction of the kinetic enolate of optically pure acetylcyclohexene with methyl crotonate. The decalone was transformed into a solanapyrone core by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service