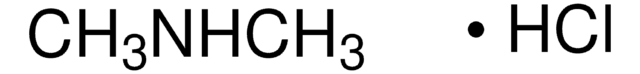126365
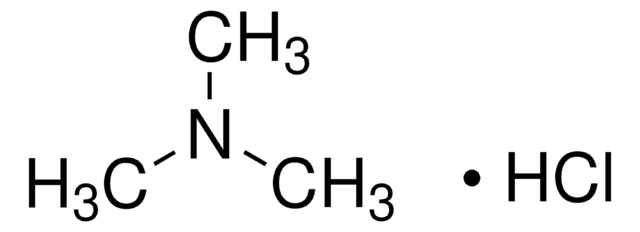
Dimethylamine hydrochloride
99%
Synonym(s):
N,N-Dimethylamine hydrochloride, N-Methylmethanamine hydrochloride, Dimethylammonium chloride
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Linear Formula:
(CH3)2NH · HCl
CAS Number:
Molecular Weight:
81.54
Beilstein:
3589311
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
170-173 °C (lit.)
solubility
H2O: very soluble
alcohol: soluble
diethyl ether: soluble
SMILES string
Cl[H].CNC
InChI
1S/C2H7N.ClH/c1-3-2;/h3H,1-2H3;1H
InChI key
IQDGSYLLQPDQDV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Dimethylamine hydrochloride has been used in the preparation of hexamethylmelamine-methyl-14C. It has also been used to prepare the standard solution of methylamine (MA), dimethylamine (DMA), trimethylamine (TMA), and trimethylamine-N-oxide (TMAO) while determing methylamines and trimethylamine-N-oxide in particulate matter.
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mark E Erupe et al.
Journal of chromatography. A, 1217(13), 2070-2073 (2010-02-26)
An ion chromatography method with non-suppressed conductivity detection was developed for the simultaneous determination of methylamines (methylamine, dimethylamine, trimethylamine) and trimethylamine-N-oxide in particulate matter air samples. The analytes were well separated by means of cation-exchange chromatography using a 3 mM
N-demethylation of the antineoplastic agent hexamethylmelamine by rats and man.
J F Worzalla et al.
Cancer research, 33(11), 2810-2815 (1973-11-01)
Mojca Seručnik et al.
The journal of physical chemistry. B, 122(21), 5381-5388 (2018-01-26)
Complexes of polycations and DNA, also known as polyplexes, have been extensively studied in the past decade, as potential gene delivery systems. Their stability depends strongly on the characteristics of the polycations, as well as the nature of the added
Gerhild Zauner et al.
Biochimica et biophysica acta, 1820(9), 1420-1428 (2011-08-02)
Analysis of protein glycosylation is an important first step towards establishing the functions of glycans in health and disease. In contrast to N-glycans which are generally enzymatically released for analysis, there is no corresponding enzyme for O-glycan liberation. Therefore, O-glycans
Lokesh Padhye et al.
Environmental science & technology, 45(10), 4353-4359 (2011-04-21)
Interactions of ozone with organic precursors during water treatment may generate carcinogenic N-nitrosodimethylamine (NDMA) byproduct. This study investigates the reaction mechanisms responsible for NDMA formation from ozonation of the commonly used poly(diallyldimethylammonium chloride) (polyDADMAC) coagulant. Upon ozonation, polyDADMAC yields the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service