106550
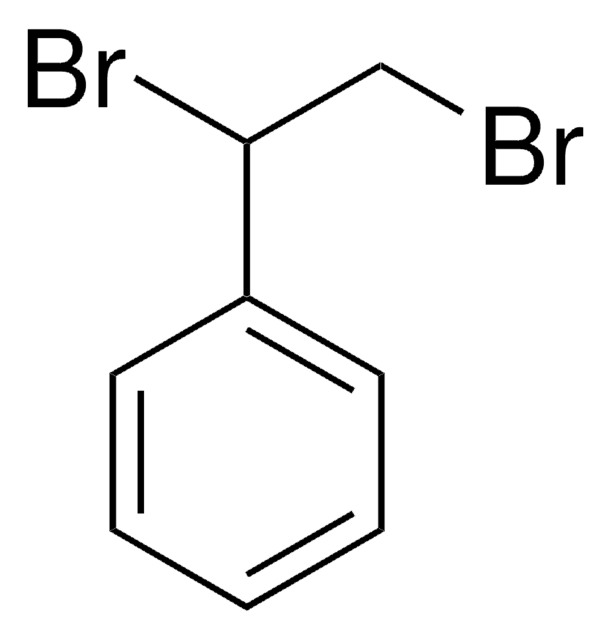
meso-1,2-Dibromo-1,2-diphenylethane
≥97%
Synonym(s):
Stilbene dibromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH(Br)CH(Br)C6H5
CAS Number:
Molecular Weight:
340.05
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
form
solid
mp
241 °C (dec.) (lit.)
functional group
bromo
phenyl
SMILES string
Br[C@H]([C@H](Br)c1ccccc1)c2ccccc2
InChI
1S/C14H12Br2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-14H/t13-,14+
InChI key
GKESIQQTGWVOLH-OKILXGFUSA-N
General description
meso-1,2-Dibromo-1,2-diphenylethane undergoes electrochemical dehalogenation in acetonitrile. It is reduced by electrogenerated C60−3.
Application
meso-1,2-Dibromo-1,2-diphenylethane(meso-SBr2) was used to study the reaction of ± and meso-SBr2 with 9-substituted fluorenide ions in dimethyl sulfoxide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Electro-organic chemistry of fullerenes. Part 1. Indirect cathodic reduction of vic-dihalides and perfluoroalkyl halides using C60 as mediator. Cyclic voltammetric study and preparative-scale electrolysis.
Fuchigami T, et al.
Journal of Electroanalytical Chemistry, 411(1), 115-119 (1996)
Debromination of meso-and (?)-1, 2-Dibromo-1, 2-diphenylethane by 9-Substituted Fluorenide Ions: Correlation between Stereochemical Results and Redox Potentials.
Lund T, et al.
Acta Chemica Scandinavica, 47, 877-877 (1993)
Reduction of Vicinal Dihalides. I. The Electrochemical Reduction of meso and (?)-1, 2-Dibromo-1, 2-diphenylethane.
Fawell P, et al.
Australian Journal of Chemistry, 43(8), 1421-1430 (1990)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service