Lateral Flow Assay Development Services

IVD assay development challenges
Developing a successful diagnostic lateral flow assay (LFA) requires significant expertise in many disciplines
- Biology & Chemistry
- Engineering
- Process Scale Up
- IVD Manufacturing
- Diagnostic Regulatory Requirements
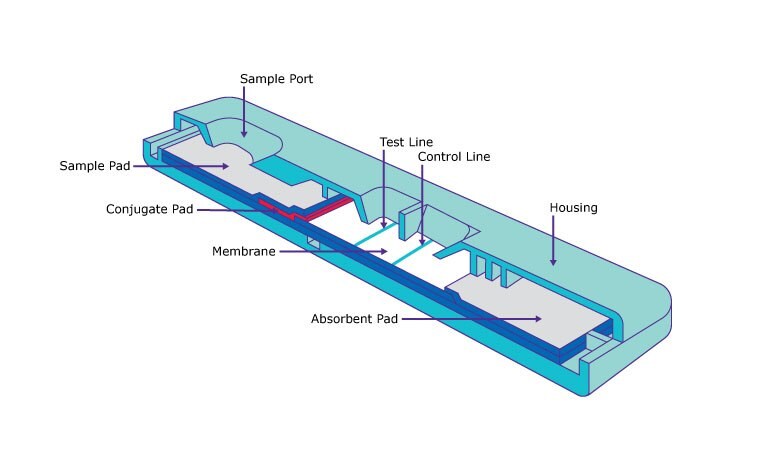
Optimizing lateral flow assay performance
Our goal is to work with you to optimize your lateral flow test for success. We provide expertise as a raw material developer, and in particular, a leader in lateral flow membranes to accelerate your IVD commercialization.
Benefits of Working With a Lateral Flow Membrane Expert
- Utilize vast knowledge of assay raw material market and kit component interactions for sourcing products
- Leverage expert lateral flow assay R&D scientists with dedicated laboratories for troubleshooting
- Avoid common IVD assay development pitfalls and accelerate commercialization
- Reduce time and supplies in optimization steps
- Work with a global partner with robust quality systems
- Expert support can increase confidence in response to regulatory challenges
We are proud to partner with the world’s largest diagnostics manufacturers as well as helping new diagnostics companies navigate compliance requirements and launch the products that will transform healthcare.
Explore our Lateral Flow Assay Development Services Capabilities
Thank you for your interest in our Lateral Flow Assay Development Services. Please complete the form below and we will contact you shortly.
Fields indicated by an * are required.
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?