推薦產品
等級
pharmaceutical primary standard
API 家族
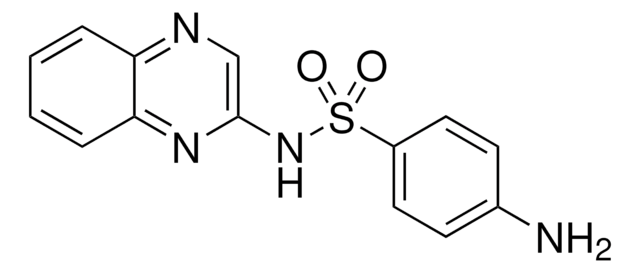
sulfaquinoxaline
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
Nc1ccc(cc1)S(=O)(=O)Nc2cnc3ccccc3n2
InChI
1S/C14H12N4O2S/c15-10-5-7-11(8-6-10)21(19,20)18-14-9-16-12-3-1-2-4-13(12)17-14/h1-9H,15H2,(H,17,18)
InChI 密鑰
NHZLNPMOSADWGC-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Sulfaquinoxaline USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Sulfaquinoxaline Oral Solution
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Resp. Sens. 1 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
Guyue Cheng et al.
Analytical and bioanalytical chemistry, 406(30), 7899-7910 (2014-10-31)
Fluoroquinolones (FQNs) are broad-spectrum antibacterial agents widely used in animal husbandry and aquaculture. The residues and antimicrobial resistance of such antibiotics are a major public health concern. To realize multianalyte detection of FQN residues, a genetically modified bacterium, Escherichia coli
Maki Kanda et al.
Journal of AOAC International, 98(1), 230-247 (2015-04-11)
Residues of 37 polar veterinary drugs belonging to six families (quinolones, tetracyclines, macrolides, lincosamides, sulfonamides, and others) in livestock and fishery products were determined using a validated LC-MS/MS method. There were two key points in sample preparation. First, extraction was
Yisheng Chen et al.
Journal of chromatography. A, 1356, 249-257 (2014-07-13)
The world-wide usage and partly abuse of veterinary antibiotics resulted in a pressing need to control residues in animal-derived foods. Large-scale screening for residues of antibiotics is typically performed by microbial agar diffusion tests. This work employing high-performance thin-layer chromatography
Yong-Gang Zhao et al.
Journal of chromatography. A, 1345, 17-28 (2014-05-02)
A novel, simple and sensitive method was developed for the simultaneous determination of 22 sulfonamides (SAs) in chicken breast muscle by using the dispersive micro-solid-phase extraction (d-μ-SPE) procedure combined with ultra-fast liquid chromatography-tandem quadrupole mass spectrometry (UFLC-MS/MS). The excellent core-shell
Alexandre Grand-Guillaume Perrenoud et al.
Journal of chromatography. A, 1360, 275-287 (2014-08-19)
Superficially porous particles (SPP), or core shell particles, which consist of a non-porous silica core surrounded by a thin shell of porous silica, have gained popularity as a solid support for chromatography over the last decade. In the present study
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務