推薦產品
等級
pharmaceutical primary standard
API 家族
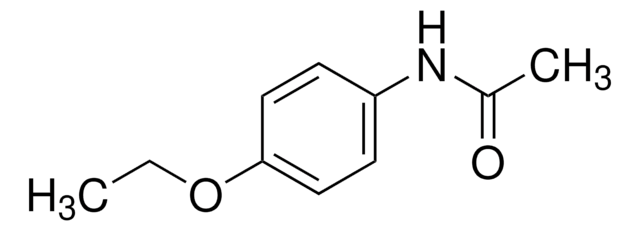
phenacetin
製造商/商標名
USP
mp
133-136 °C (lit.)
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
CCOc1ccc(NC(C)=O)cc1
InChI
1S/C10H13NO2/c1-3-13-10-6-4-9(5-7-10)11-8(2)12/h4-7H,3H2,1-2H3,(H,11,12)
InChI 密鑰
CPJSUEIXXCENMM-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Phenacetin Melting Point Standard (Phenacetin) is a lipid-soluble drug with moderate solubility with aqueous solvents. Its oral absorption is based on formulation factors like particle size. Its major metabolic route is O-dealkylation to paracetamol and its minor pathways are deacetylation and hydroxylation to form pphenetidine, 2-hydroxyphenetidine, 2- and 3-hydroxyphenacetin and N-hydroxyphenacetin.
應用
Phenacetin Melting Point Standard USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
生化/生理作用
CYP1A2 和 CYP2D6 的底物。
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Carc. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Kinetics and metabolism of paracetamol and phenacetin
Prescott, L.F.
British Journal of Clinical Pharmacology (1980)
V M Buckalew
American journal of kidney diseases : the official journal of the National Kidney Foundation, 28(1 Suppl 1), S7-13 (1996-07-01)
Six epidemiologic studies in the United States and Europe indicate that habitual use of phenacetin is associated with the development of chronic renal failure and end-stage renal disease (ESRD), with a relative risk in the range of 4 to 19.
J A Hinson
Environmental health perspectives, 49, 71-79 (1983-03-01)
Phenacetin can be metabolized to reactive metabolites by a variety of mechanisms. (1) Phenacetin can be N-hydroxylated, and the resulting N-hydroxyphenacetin can be sulfated or glucuronidated. Whereas phenacetin N-O sulfate immediately rearranges to form a reactive metabolite which may covalently
Phenacetin abuse: a review.
G Carro-Ciampi
Toxicology, 10(4), 311-339 (1978-08-01)
L F Prescott
British journal of clinical pharmacology, 10 Suppl 2, 291S-298S (1980-10-01)
1 The rate of absorption of oral paracetamol depends on the rate of gastric emptying and is usually rapid and complete. The mean systemic availability is about 75%. 2 Paracetamol is extensively metabolized and the plasma half-life is 1.5-2.5 hours.
條款
US EPA Method 8270 (Appendix IX): GC Analysis of Semivolatiles on Equity®-5 (30 m x 0.25 mm I.D., 0.50 μm)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務