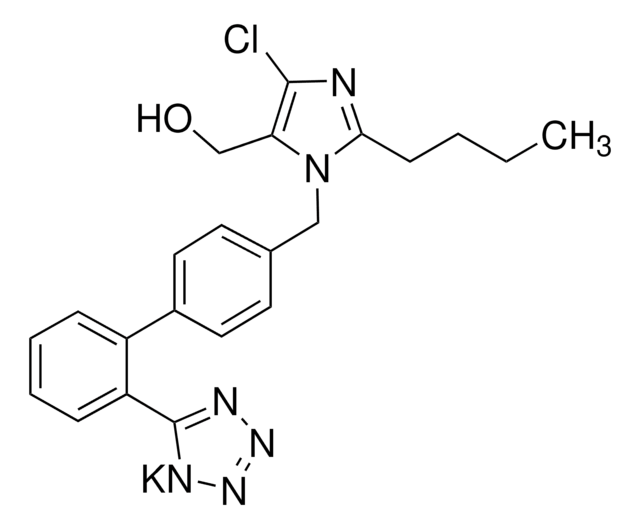
推薦產品
等級
pharmaceutical primary standard
API 家族
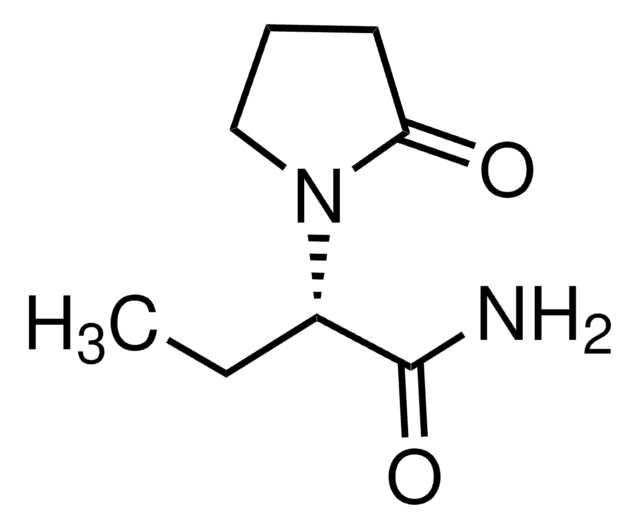
levetiracetam
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
CC[C@H](N1CCCC1=O)C(N)=O
InChI
1S/C8H14N2O2/c1-2-6(8(9)12)10-5-3-4-7(10)11/h6H,2-5H2,1H3,(H2,9,12)/t6-/m0/s1
InChI 密鑰
HPHUVLMMVZITSG-LURJTMIESA-N
基因資訊
human ... CACNA1B(774) , SV2A(9900)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Levetiracetam USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
生化/生理作用
左乙拉西坦是一种具有抗癫痫活性的吡咯烷。
左乙拉西坦是一种具有抗癫痫活性的吡咯烷。左乙拉西坦的立体选择性结合仅限于中枢神经系统的突触质膜,而在周围组织中不发生立体选择性结合。左乙拉西坦在不影响正常神经元兴奋性的情况下抑制簇状放电,这表明它可以选择性地阻止癫痫样簇状放电的超同步和发作活动的传播。
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Gashirai K Mbizvo et al.
The Cochrane database of systematic reviews, 9(9), CD001901-CD001901 (2012-09-14)
Epilepsy is an important neurological condition and drug resistance in epilepsy is particularly common in individuals with focal seizures. In this review, we summarise the current evidence regarding a new antiepileptic drug, levetiracetam, when used as add-on treatment for controlling
Benjamin W Y Lo et al.
The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques, 38(3), 475-486 (2011-04-26)
New evidence suggests that levetiracetam may be as effective as traditional agents, with better safety profile. To synthesize evidence regarding efficacy and tolerability of levetiracetam as first line, adjunctive or prophylactic antiepileptic agent. Study Selection & Data Extraction: Eligible studies
Brian Longo et al.
The Annals of pharmacotherapy, 43(10), 1692-1695 (2009-08-20)
To review data evaluating levetiracetam management of epilepsy during pregnancy. A literature search of PubMed (1966-June 2009) was performed using the terms pregnancy, epilepsy, levetiracetam, and anticonvulsants. Bibliographies of all articles retrieved were reviewed to identify additional relevant articles. All
Amy Z Crepeau et al.
Expert review of neurotherapeutics, 10(2), 159-171 (2010-02-09)
Levetiracetam was approved by the US FDA in 1999 after failing the traditional screening tests for antiepileptic drugs. In the 10 years since its introduction, it has become one of the first-line antiepileptic agents and has been evaluated in small
Vincenzo Belcastro et al.
Brain & development, 33(4), 289-293 (2010-07-16)
Several new antiepileptic drugs (AEDs) have been introduced for clinical use recently. These new AEDs, like the classic AEDs, target multiple cellular sites both pre- and postsynaptically. The use of AEDs as a possible neuroprotective strategy in brain ischemia is
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務