推薦產品
等級
pharmaceutical primary standard
API 家族
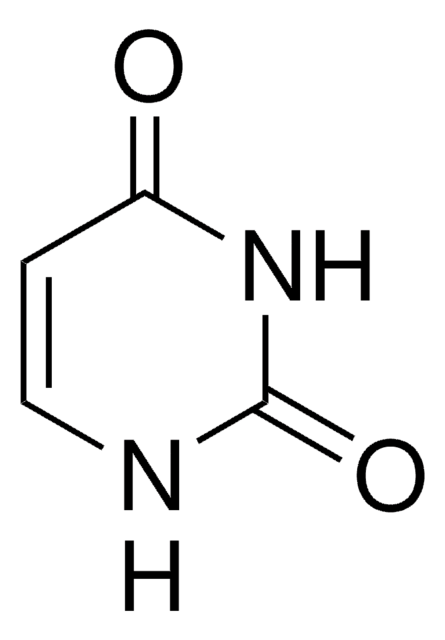
fluorouracil
製造商/商標名
USP
mp
282-286 °C (dec.) (lit.)
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
FC1=CNC(=O)NC1=O
InChI
1S/C4H3FN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9)
InChI 密鑰
GHASVSINZRGABV-UHFFFAOYSA-N
基因資訊
human ... TYMS(7298)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Fluorouracil USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Flucytosine
- Flucytosine Capsules
- Fluorouracil
- Fluorouracil Cream
- Fluorouracil Injection
- Fluorouracil Topical Solution
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Carc. 2
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Zhe Guo et al.
Annals of surgical oncology, 21(9), 3069-3076 (2014-04-15)
It is unclear whether hepatic resection (HR) or transarterial chemoembolization (TACE) is associated with better outcomes for patients with hepatocellular carcinoma (HCC) in Barcelona Clinic Liver Cancer (BCLC) stage A. The present study compared survival for patients with BCLC stage
Tania Diaz et al.
Journal of surgical oncology, 109(7), 676-683 (2014-02-11)
Surgery is the standard treatment for colorectal cancer (CRC), and adjuvant chemotherapy has been shown to be effective in stage III but less so in stage II. We have analyzed the expression of the miR-200 family in tissue samples from
John M L Ebos et al.
EMBO molecular medicine, 6(12), 1561-1576 (2014-11-02)
Thousands of cancer patients are currently in clinical trials evaluating antiangiogenic therapy in the neoadjuvant setting, which is the treatment of localized primary tumors prior to surgical intervention. The rationale is that shrinking a tumor will improve surgical outcomes and
Sharlene Gill et al.
Cancer treatment reviews, 40(10), 1171-1181 (2014-12-03)
Colorectal cancer (CRC) is the third most commonly diagnosed cancer among males and second among females worldwide. The treatment landscape for advanced CRC (aCRC) is rapidly evolving and there are now a number of randomized trials assessing treatment of aCRC
Comparative economics of a 12-gene assay for predicting risk of recurrence in stage II colon cancer.
Steven R Alberts et al.
PharmacoEconomics, 32(12), 1231-1243 (2014-08-27)
Prior economic analysis that compared the 12-gene assay to published patterns of care predicted the assay would improve outcomes while lowering medical costs for stage II, T3, mismatch-repair-proficient (MMR-P) colon cancer patients. This study assessed the validity of those findings
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務