推薦產品
等級
pharmaceutical primary standard
API 家族
acetaminophen, paracetamol
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
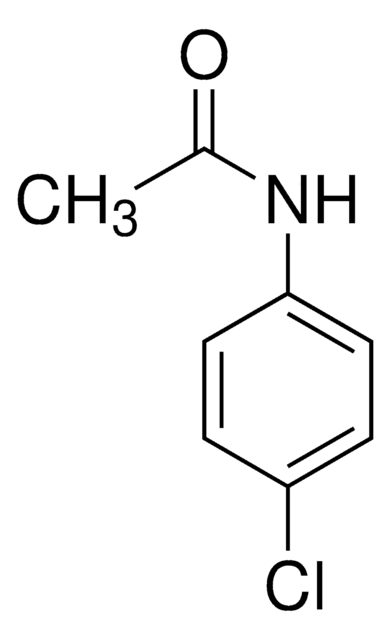
N(c1ccc(cc1)OC(=O)C)C(=O)C
InChI
1S/C10H11NO3/c1-7(12)11-9-3-5-10(6-4-9)14-8(2)13/h3-6H,1-2H3,(H,11,12)
InChI 密鑰
UJAOSPFULOFZRR-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Acetaminophen Related Compound A USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
[N,O-diacetyl-p-aminophenol formation by combined use of aspirin with phenacetin and acetaminophen in vivo (author's transl)].
Y N Kyo et al.
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 100(10), 1043-1047 (1980-10-01)
R Thomis et al.
Journal of pharmaceutical sciences, 73(12), 1830-1833 (1984-12-01)
The high-performance liquid chromatographic method described enables the quantitation of the components and the main impurities of tablets containing aspirin, acetaminophen, and ascorbic acid. A C8 reverse-phase column was used; the mobile phase was methanol-0.2 M phosphate buffer (pH 3.5)-water
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務